{beta}-glucanase Eng2 is required for ascus wall endolysis after sporulation in the fission yeast Schizosaccharomyces pombe - PubMed (original) (raw)
{beta}-glucanase Eng2 is required for ascus wall endolysis after sporulation in the fission yeast Schizosaccharomyces pombe
Javier Encinar del Dedo et al. Eukaryot Cell. 2009 Aug.
Abstract
Meiosis is the developmental program by which sexually reproducing diploid organisms generate haploid gametes. In yeast, meiosis is followed by spore morphogenesis. When Schizosaccharomyces pombe diploid cells undergo meiosis, they differentiate into asci containing four haploid ascospores that are highly resistant to environmental stress. The formation of the ascospore wall requires the activity of several enzymes involved in the biosynthesis and modification of its components, such as alpha- and beta-glucan synthases. Once the spores are completely mature, the wall of the ascus undergoes an endolytic process that results in the release of ascospores from the ascus, allowing their dispersal into the environment. This process requires the activity of the endo-alpha-1,3-glucanase Agn2. Here, we focus on the characterization of the endo-beta-1,3-glucanase Eng2, which is also required for ascospore release from the ascus. Although Eng2 is present during the mitotic cycle, the protein accumulates after meiosis II. The expression of eng2(+) is required for the efficient release of ascospores, as shown by placing eng2(+) under the control of a repressible promoter. Furthermore, a point mutation that destroys the catalytic activity of the protein results in a phenotype similar to that of the mutant strain. Finally, we demonstrate that exogenous addition of purified Eng2 releases the ascospores from asci generated by an eng2Delta mutant. We propose that Eng2 would act together with Agn2 to completely hydrolyze the ascus wall, thereby assisting in the release of ascospores in S. pombe.
Figures
FIG. 1.
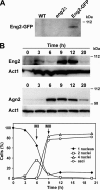
Eng2 accumulates during sporulation. (A) Western blot analysis of Eng2-GFP during vegetative growth. Samples were collected from exponentially growing cultures of wild-type (WT; OL23), _eng2_Δ mutant (OL24), and _eng2_-GFP (OL896) strains to prepare protein extracts. Anti-GFP antibody was used. (B) Western blot analysis of Eng2-GFP and Agn2-GFP during sporulation. The diploid strains OL961 and OL962 were induced to sporulate. Samples were collected at the indicated times after the induction of sporulation to prepare protein extracts. Anti-GFP antibody was used. Actin was used as a loading control. Meiotic progression was followed by DAPI staining of nuclei, and sporulation was checked by microscopic observation of asci. The percentages of mononucleate, binucleate, and tetranucleate cells and spores at each time point for the strain carrying Eng2-GFP are shown in the graph.
FIG. 2.
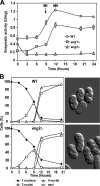
β-1,3-Glucanase activity during sporulation. (A) The diploid strains OL946 (WT), OL948 (_eng1_Δ/_eng1_Δ), and OL950 (_eng2_Δ/_eng2_Δ) were grown on EMM-AC and then transferred to EMM-SG to induce sporulation. Samples were collected at the indicated times after the induction of sporulation to prepare protein extracts and to assay β-1,3-glucanase activity, using laminarin as substrate. Activity is represented as units/mg protein. Values are means of results from three independent measurements, and standard deviations are shown. MI, meiosis I; MII, meiosis II. (B) Meiotic progression of the wild-type (WT) and _eng2_Δ/_eng2_Δ strains. Aliquots of the culture were stained with DAPI, and the percentages of mononucleate, binucleate, and tetranucleate cells and spores at each time-point are shown. Images show mature spores after 24 h of incubation in sporulation medium.
FIG. 3.
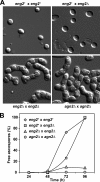
Eng2 participates in ascus wall hydrolysis following sporulation. (A) Microscopic appearance of sporulated cultures obtained from crosses between wild-type haploid (OL176/OL177), eng2+/_eng2_Δ (OL176/OL773), _eng2_Δ/_eng2_Δ (OL759/OL773), and _agn2_Δ/_agn2_Δ (OL763/OL777) strains. Cultures were incubated for 96 h before the images were taken. (B) Quantification of ascospore release from the asci indicated in sporulated cultures from the same crosses. At the indicated time intervals, the percentages of free ascospores were determined by light microscopy. At least 150 cells were counted for each time point.
FIG. 4.
Expression of eng2+ is essential for the release of ascospores from asci. The haploid _eng2_Δ strain carrying P_nmt1_-eng2 (OL958) and the haploid strains from the opposite mating type, OL176 (WT) and OL773 (_eng2_Δ), were grown on YES medium to mid-log phase. Equal numbers of cells were collected and spotted onto EMM-N plates with (+T) and without (−T) thiamine to induce mating and sporulation. At the indicated times, aliquots were collected for microscopic inspection and quantification of the percentage of free spores. The crosses were eng2+/_eng2_Δ (WT), _eng2_Δ/_eng2_Δ (_eng2_Δ), and _eng2_Δ/_eng2_Δ plus P_nmt1_-eng2 (HA-eng2). (A) Western analysis of Eng2-HA after 24 of incubation in EMM-N medium. (B) Percentages of free spores. (C) Sample images of the different strains grown in the presence and absence of thiamine.
FIG. 5.
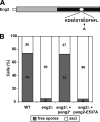
Eng2 catalytic activity is required for ascus endolysis. (A) Schematic representation of Eng2. The gray rectangle indicates the common region present in GH81 proteins that contains the putative catalytic domain of the protein (black rectangle). The white circle marks the position of the two perfectly conserved Glu residues, which have been proposed to act as putative nucleophiles (asterisks). E537 was mutated to Ala. (B) Quantification of ascospore release from the asci in sporulated cultures. At the indicated time intervals, the percentages of free ascospores were determined by light microscopy. At least 150 cells were counted for each time point. The crosses were OL176/OL177 (WT), OL759/OL773 (_eng2_Δ), OL759/OL773 carrying pJED12 (eng2_Δ/p_eng2+), and OL759/OL773 carrying pJED13 [eng2_Δ/p_eng2(E537A)].
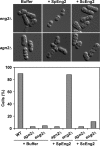
FIG. 6.
Exogenous addition of purified Eng2 results in ascospore release. _eng2_Δ/_eng2_Δ (OL759/OL773) or _agn2_Δ/_agn2_Δ (OL763/OL777) diploid cells were allowed to sporulate for 7 days. After spore formation, the asci were incubated for 60 min at 37°C with buffer or 0.05 units of purified S. pombe Eng2 (SpEng2) or S. cerevisiae Eng2 (ScEng2). The percentage of ascospores released in each culture was examined by light microscopy. At least 100 cells were counted. WT, wild type.
FIG. 7.
Eng2 localizes to the cytoplasm of the ascus. (A) Haploid Eng2-GFP (OL952) or Agn2-GFP (OL954) cells were allowed to mate on sporulation plates with cells from the opposite mating type carrying the same tagged proteins (strain OL953 or OL955, respectively), and the resulting zygotic asci were examined using fluorescence microscopy. Bar, 10 μm. (B) Details of free spores. (C) Germination of spores from the wild-type (WT) and _eng2_Δ/_eng2_Δ crosses. DIC, differential interface contrast.
Similar articles
- Sporulation: A response to starvation in the fission yeast Schizosaccharomyces pombe.
Ohtsuka H, Imada K, Shimasaki T, Aiba H. Ohtsuka H, et al. Microbiologyopen. 2022 Jun;11(3):e1303. doi: 10.1002/mbo3.1303. Microbiologyopen. 2022. PMID: 35765188 Free PMC article. Review. - Role of the alpha-glucanase Agn2p in ascus-wall endolysis following sporulation in fission yeast.
Dekker N, van Rijssel J, Distel B, Hochstenbach F. Dekker N, et al. Yeast. 2007 Apr;24(4):279-88. doi: 10.1002/yea.1464. Yeast. 2007. PMID: 17315266 - The beta-1,3-glucanosyltransferase gas4p is essential for ascospore wall maturation and spore viability in Schizosaccharomyces pombe.
de Medina-Redondo M, Arnáiz-Pita Y, Fontaine T, Del Rey F, Latgé JP, Vázquez de Aldana CR. de Medina-Redondo M, et al. Mol Microbiol. 2008 Jun;68(5):1283-99. doi: 10.1111/j.1365-2958.2008.06233.x. Epub 2008 Apr 8. Mol Microbiol. 2008. PMID: 18410286 - Bgs2p, a 1,3-beta-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe.
Liu J, Tang X, Wang H, Balasubramanian M. Liu J, et al. FEBS Lett. 2000 Jul 28;478(1-2):105-8. doi: 10.1016/s0014-5793(00)01828-7. FEBS Lett. 2000. PMID: 10922478 - Analysis of Schizosaccharomyces pombe Meiosis.
Yamashita A, Sakuno T, Watanabe Y, Yamamoto M. Yamashita A, et al. Cold Spring Harb Protoc. 2017 Sep 1;2017(9):pdb.top079855. doi: 10.1101/pdb.top079855. Cold Spring Harb Protoc. 2017. PMID: 28733417 Review.
Cited by
- Global coordination of transcriptional control and mRNA decay during cellular differentiation.
Amorim MJ, Cotobal C, Duncan C, Mata J. Amorim MJ, et al. Mol Syst Biol. 2010 Jun 8;6:380. doi: 10.1038/msb.2010.38. Mol Syst Biol. 2010. PMID: 20531409 Free PMC article. - Sporulation: A response to starvation in the fission yeast Schizosaccharomyces pombe.
Ohtsuka H, Imada K, Shimasaki T, Aiba H. Ohtsuka H, et al. Microbiologyopen. 2022 Jun;11(3):e1303. doi: 10.1002/mbo3.1303. Microbiologyopen. 2022. PMID: 35765188 Free PMC article. Review. - Glucanase-Induced Stipe Wall Extension Shows Distinct Differences from Chitinase-Induced Stipe Wall Extension of Coprinopsis cinerea.
Kang L, Zhou J, Wang R, Zhang X, Liu C, Liu Z, Yuan S. Kang L, et al. Appl Environ Microbiol. 2019 Oct 16;85(21):e01345-19. doi: 10.1128/AEM.01345-19. Print 2019 Nov 1. Appl Environ Microbiol. 2019. PMID: 31444203 Free PMC article. - Diverse mating phenotypes impact the spread of wtf meiotic drivers in Schizosaccharomyces pombe.
López Hernández JF, Helston RM, Lange JJ, Billmyre RB, Schaffner SH, Eickbush MT, McCroskey S, Zanders SE. López Hernández JF, et al. Elife. 2021 Dec 13;10:e70812. doi: 10.7554/eLife.70812. Elife. 2021. PMID: 34895466 Free PMC article. - A quality control mechanism linking meiotic success to release of ascospores.
Guo H, King MC. Guo H, et al. PLoS One. 2013 Dec 2;8(12):e82758. doi: 10.1371/journal.pone.0082758. eCollection 2013. PLoS One. 2013. PMID: 24312672 Free PMC article.
References
- Arellano, M., H. Cartagena-Lirola, M. A. Nasser Hajibagheri, A. Durán, and M. H. Valdivieso. 2000. Proper ascospore maturation requires the chs1+ chitin synthase gene in Schizosaccharomyces pombe. Mol. Microbiol. 3579-89. - PubMed
- Bähler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14943-951. - PubMed
- Dekker, N., A. de Haan, and F. Hochstenbach. 2006. Transcription regulation of the α-glucanase gene agn1 by cell separation transcription factor Ace2p in fission yeast. FEBS Lett. 5803099-3106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases