Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis - PubMed (original) (raw)
Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis
Courtney M Karner et al. Nat Genet. 2009 Jul.
Abstract
Although many vertebrate organs, such as kidneys, lungs and liver, are composed of epithelial tubules, little is known of the mechanisms that establish the length or diameter of these tubules. In the kidney, defects in the establishment or maintenance of tubule diameter are associated with one of the most common inherited human disorders, polycystic kidney disease. Here we show that attenuation of Wnt9b signaling during kidney morphogenesis affects the planar cell polarity of the epithelium and leads to tubules with significantly increased diameter. Although previous studies showed that polarized cell divisions maintain the diameter of postnatal kidney tubules, we find that cell divisions are randomly oriented during embryonic development. Our data suggest that diameter is established during early morphogenetic stages by convergent extension processes and maintained by polarized cell divisions. Wnt9b, signaling through the non-canonical Rho/Jnk branch of the Wnt pathway, is necessary for both of these processes.
Figures
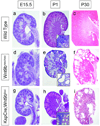
Figure 1. Defects in Wnt9b signaling results in cyst formation
H&E sections of wild type (a–c), Wnt9bneo/neo (d–f), and KspCre;Wnt9bflox/− (g–i) kidneys at embryonic day 15.5 (a,d and g), postnatal day 1 (b, e and h) or postnatal day 30 (c, f and i). Wnt9b mutant kidneys appear normal at E15.5 (compare d and g to a) but are smaller at birth (compare e and h to b). Wnt9bcneo/cneo kidneys also show signs of cystic dysplasia at P1 (e). At one month of age mutant kidneys are slightly smaller and severely cystic compared to wild type kidneys (compare f and i to c). Insets in B, E and H show high magnification images of cortical epithelia.
Figure 2. Characterization of cyst origin in Wnt9bneo/neo kidneys
Sections of P1 (a, b, e, f, i, j) and P15 (c, d, g, h, k, l) kidneys stained with the collecting duct specific marker Dolichos biflorus agglutinin (DBA) in a–d, the loop of Henle marker Tamm Horsfall protein (THP) in e–h, and the proximal tubule marker Lotus tetragolonobus lectin (LTL) in i–l. In all panels arrows denote normal tubules and asterisks denote cystic tubules. At birth, cysts are found primarily in the proximal tubules (compare i to j). Cysts are also found in the collecting ducts although the majority of DBA positive epithelia appear normal (see arrows in b). Cysts were not observed in the loop of Henle at birth (compare e to f). By P15 cysts are present in all segments of the nephron (compare c to b, g to h, and k to l). Nuclei were counterstained with DAPI (blue).
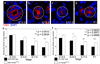
Figure 3. Cell division becomes oriented after birth in a Wnt9b–dependent process
(a) Graphical representation of the angle between the mitotic spindles and the longitudinal axis of DBA-positive tubules at E15.5 indicates that cell division in both wild type (black bars) and Wnt9bneo/neo tubules (white bars) is randomly oriented at E15.5 when compared to the expected random distribution by the Kolmogorov-Smirnov (KS) test. P > 0.55 for both wild type (N=109) and mutant (n=96). (b) At P5, the orientation of dividing cells in KspCre;Wnt9b−/flox DBA positive cells (white bars, n=50) is significantly different (p < 0.01, Mann-Whitney U test) from wild type (black bars, n=45) indicating that Wnt9b is necessary for orientation of cell division that occurs post-natally.
Figure 4. Wnt9b is required for the elongation and narrowing of kidney tubules
Representative sections through wild type DBA positive tubules from E13.5 (a), E15.5 (b), E17.5 (c), and P1 mice (d) showing the number of nuclei composing the wall of the tubule. Outlined tubules represent transverse sections. Quantitation reveals that the number of cells within the wall of wild-type collecting duct (black bars in e, n=563, 606, 844, and 692 for E13.5, 15.5, 17.5 and P1 respectively) and proximal tubules (black bars in f, n=425, 1030, 791, and 778 for E13.5, 15.5, 17.5 and P1 respectively) significantly decreases during the embryonic period. The number of cells within the tubule wall is significantly increased in Wnt9b mutant kidneys (white bars in e and f, n=384 or 412, 521 or 424, 915 or 902, and 665 or 635 for DBA or LTL at E13.5, 15.5 17.5 or P1 respectively). N=3 kidneys for each stage, tubular segment and genotype. Error bars represent standard error of the mean.
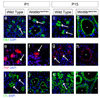
Figure 5
Wnt9b is necessary for the orientation of polarized cells perpendicular to the axis of extension. Confocal images (a and d), cell outlines (b and e) and 3D reconstructions (c and f) of frontal sections through E15.5 wild type (a–c) and Wnt9bneo/neo (d–f) kidneys stained with anti-E-cadherin (green), anti-aPKC (red) and DBA (blue). In all cases, proximal is up and distal is down. The images in a and d represent sections just basal to the apical membrane as marked by anti-aPKC (red, not seen). Mediolaterally elongated cells are marked in white, proximal-distally elongated cells in black and unelongated cells in gray in b and e. The majority of wild-type cells are seen to be mediolaterally elongated perpendicular to the axis of extension (white in b). Wnt9bneo/neo cells are still elongated but the direction of elongation appears to be random (note increased number of black, proximal-distally elongated cells in e relative to b). (c and f) 3D reconstructions of Wild type (c) or Wnt9bneo/neo (f) e15.5 tubules to allow for visualization of cell orientation. Arrows indicate angle of orientation for marked cells. Quantitation of the angle of cellular elongation relative to the proximal distal axis of the tubule for wild-type (left in g and h) and Wnt9bneo/neo mutant (right in g) or KspCre;Wnt9b−/flox (right in h) cells. White bars indicate cells that are perpendicular to the axis of elongation (45–90°) while black bars represent cells that are parallel (0–45°). The percentage of cells within each 10 degree increment is indicated. There is a significant change in the orientation of the elongated cells between wild type and mutants. (P<0.001, KS test). The data was gathered from at least 3 different animals. The total number of oriented cells analyzed is 92, 86, or 93 for wild type, KspCre;Wnt9bflox/−, or Wnt9b neo/neo respectively. Wild type cells are from littermate controls.
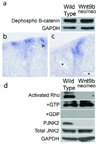
Figure 6. Wnt9b signals through the noncanonical pathway to regulate tubule diameter
Western blots of total protein extracted from wild-type and Wnt9bneo/neo kidneys probed with an antibody specific to the dephosphorylated (active) form of β-catenin show no significant differences in canonical Wnt activity compared to wild type (a). Section in situ hybridization with a probe for the β−catenin target axin-2 also shows no significant decrease in canonical activity in P1 Wnt9bneo/neo kidneys (c) compared to wild type (b). Note that there is no ectopic axin2 expression in cystic proximal tubules (asterisks in c). (d) Western blots indicate that activated Rho is significantly decreased in Wnt9bneo/neo kidneys at P1 relative to total (+GTP control) Rho levels. Addition of GDP (+GDP) to inactivate Rho was used as a negative control. Phosphorylated Jnk2 is also significantly decreased in the Wnt9bneo/neo kidneys at P1 relative to total levels of Jnk2 (d). Blots shown are representative examples of data gathered from at least 3 different blots from 3 independent protein extractions.
Similar articles
- The kidney and planar cell polarity.
Carroll TJ, Yu J. Carroll TJ, et al. Curr Top Dev Biol. 2012;101:185-212. doi: 10.1016/B978-0-12-394592-1.00011-9. Curr Top Dev Biol. 2012. PMID: 23140630 Free PMC article. Review. - Disruption of Core Planar Cell Polarity Signaling Regulates Renal Tubule Morphogenesis but Is Not Cystogenic.
Kunimoto K, Bayly RD, Vladar EK, Vonderfecht T, Gallagher AR, Axelrod JD. Kunimoto K, et al. Curr Biol. 2017 Oct 23;27(20):3120-3131.e4. doi: 10.1016/j.cub.2017.09.011. Epub 2017 Oct 12. Curr Biol. 2017. PMID: 29033332 Free PMC article. - Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension.
Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G. Lienkamp SS, et al. Nat Genet. 2012 Dec;44(12):1382-7. doi: 10.1038/ng.2452. Epub 2012 Nov 11. Nat Genet. 2012. PMID: 23143599 Free PMC article. - Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways.
Happé H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ. Happé H, et al. Hum Mol Genet. 2009 Jul 15;18(14):2532-42. doi: 10.1093/hmg/ddp190. Epub 2009 Apr 28. Hum Mol Genet. 2009. PMID: 19401297 - Planar cell polarity of the kidney.
Schnell U, Carroll TJ. Schnell U, et al. Exp Cell Res. 2016 May 1;343(2):258-266. doi: 10.1016/j.yexcr.2014.11.003. Epub 2014 Nov 13. Exp Cell Res. 2016. PMID: 25445789 Free PMC article. Review.
Cited by
- Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease.
Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Boulter L, et al. Nat Med. 2012 Mar 4;18(4):572-9. doi: 10.1038/nm.2667. Nat Med. 2012. PMID: 22388089 Free PMC article. - Signaling during Kidney Development.
Krause M, Rak-Raszewska A, Pietilä I, Quaggin SE, Vainio S. Krause M, et al. Cells. 2015 Apr 10;4(2):112-32. doi: 10.3390/cells4020112. Cells. 2015. PMID: 25867084 Free PMC article. Review. - mTOR and S6K1 drive polycystic kidney by the control of Afadin-dependent oriented cell division.
Bonucci M, Kuperwasser N, Barbe S, Koka V, de Villeneuve D, Zhang C, Srivastava N, Jia X, Stokes MP, Bienaimé F, Verkarre V, Lopez JB, Jaulin F, Pontoglio M, Terzi F, Delaval B, Piel M, Pende M. Bonucci M, et al. Nat Commun. 2020 Jun 24;11(1):3200. doi: 10.1038/s41467-020-16978-z. Nat Commun. 2020. PMID: 32581239 Free PMC article. - Wnt9b-dependent FGF signaling is crucial for outgrowth of the nasal and maxillary processes during upper jaw and lip development.
Jin YR, Han XH, Taketo MM, Yoon JK. Jin YR, et al. Development. 2012 May;139(10):1821-30. doi: 10.1242/dev.075796. Epub 2012 Mar 29. Development. 2012. PMID: 22461561 Free PMC article. - Preparing for the first breath: genetic and cellular mechanisms in lung development.
Morrisey EE, Hogan BL. Morrisey EE, et al. Dev Cell. 2010 Jan 19;18(1):8-23. doi: 10.1016/j.devcel.2009.12.010. Dev Cell. 2010. PMID: 20152174 Free PMC article. Review.
References
References for Methods
References
- Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 2007;8:791–802. - PubMed
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. - PubMed
- Ibraghimov-Beskrovnaya O. Targeting dysregulated cell cycle and apoptosis for polycystic kidney disease therapy. Cell Cycle. 2007;6:776–779. - PubMed
- Araujo SJ, Aslam H, Tear G, Casanova J. mummy/cystic encodes an enzyme required for chitin and glycan synthesis, involved in trachea, embryonic cuticle and CNS development--analysis of its role in Drosophila tracheal morphogenesis. Dev Biol. 2005;288:179–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5 P30 DK07403802/DK/NIDDK NIH HHS/United States
- P30 DK074038/DK/NIDDK NIH HHS/United States
- 1 R01 DK080004/DK/NIDDK NIH HHS/United States
- P30 DK074038-01/DK/NIDDK NIH HHS/United States
- R01 DK080004/DK/NIDDK NIH HHS/United States
- R37 DK042921/DK/NIDDK NIH HHS/United States
- P30 DK079328/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous