Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1) - PubMed (original) (raw)
Review
Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1)
Brian J Coburn et al. BMC Med. 2009.
Abstract
Here we present a review of the literature of influenza modeling studies, and discuss how these models can provide insights into the future of the currently circulating novel strain of influenza A (H1N1), formerly known as swine flu. We discuss how the feasibility of controlling an epidemic critically depends on the value of the Basic Reproduction Number (R0). The R0 for novel influenza A (H1N1) has recently been estimated to be between 1.4 and 1.6. This value is below values of R0 estimated for the 1918-1919 pandemic strain (mean R0 approximately 2: range 1.4 to 2.8) and is comparable to R0 values estimated for seasonal strains of influenza (mean R0 1.3: range 0.9 to 2.1). By reviewing results from previous modeling studies we conclude it is theoretically possible that a pandemic of H1N1 could be contained. However it may not be feasible, even in resource-rich countries, to achieve the necessary levels of vaccination and treatment for control. As a recent modeling study has shown, a global cooperative strategy will be essential in order to control a pandemic. This strategy will require resource-rich countries to share their vaccines and antivirals with resource-constrained and resource-poor countries. We conclude our review by discussing the necessity of developing new biologically complex models. We suggest that these models should simultaneously track the transmission dynamics of multiple strains of influenza in bird, pig and human populations. Such models could be critical for identifying effective new interventions, and informing pandemic preparedness planning. Finally, we show that by modeling cross-species transmission it may be possible to predict the emergence of pandemic strains of influenza.
Figures
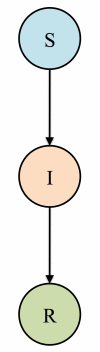
Figure 1
Compartmental SIR model of disease transmission. The population is partitioned into three classes: Susceptible (S), Infectious (I), and Recovered (R). Individuals who become infected proceed from class S to class I at a rate which depends on the infectiousness of the virus and the prevalence of infection. Infectious individuals recover and move to class R, at which point they are immune to future infection. The model can be straightforwardly extended to include immunity which wanes over time.
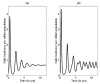
Figure 2
Daily incidence for an influenza outbreak calculated using an SIR model. (a) With constant transmission rate. (b) With small seasonal variation in transmission rate. For a constant transmission rate, after an initial transient period, the system approaches an endemic level (that is, equilibrium) by damped oscillations. If a small amount of seasonal variation in transmission is introduced oscillations are sustained rather than damping out, and the system eventually tends to an annual cycle.
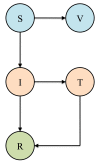
Figure 3
SIR compartmental model of disease transmission incorporating vaccination and treatment. Susceptible individuals (S) who are vaccinated proceed to class V, at which point they are considered immune. Upon treatment, infectious individuals (I) proceed to class T, at which point their infectiousness is reduced.
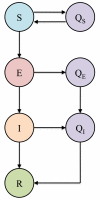
Figure 4
SEIR compartmental model of infection incorporating quarantine and isolation measures. The class E represents a latent class during which an individual who has been exposed to the pathogen is not yet infectious and is asymptomatic. Individuals who might have been exposed (S and E) are quarantined and proceed to the respective Q classes: QS and QE. When susceptible individuals in quarantine (QS) are determined not to have been infected they are returned to the susceptible class (S). Those in quarantine who develop infection (QE) are isolated and proceed to class (QI), as do infected individuals (I).
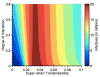
Figure 5
Results from a cross-species multi-strain transmission model where pigs act as 'mixing vessels'. This figure shows the severity of the epidemic that occurs when a 'super-strain' emerges into the human population from pigs, as a function of the cross-species interaction and the transmissibility/infectivity of the 'super-strain' in humans. The maximal number of infectives that could occur in any year (estimated over a 150-year time period) is shown in dark red. The contour map illustrates that the greatest outbreak occurs when the transmissibility/infectivity of the 'super-strain' is greater than 0.024 and less than 0.04; this implies 2.3 <_R_0 < 3.8.
Similar articles
- Seasonal transmission potential and activity peaks of the new influenza A(H1N1): a Monte Carlo likelihood analysis based on human mobility.
Balcan D, Hu H, Goncalves B, Bajardi P, Poletto C, Ramasco JJ, Paolotti D, Perra N, Tizzoni M, Van den Broeck W, Colizza V, Vespignani A. Balcan D, et al. BMC Med. 2009 Sep 10;7:45. doi: 10.1186/1741-7015-7-45. BMC Med. 2009. PMID: 19744314 Free PMC article. - Potential for a global dynamic of Influenza A (H1N1).
Flahault A, Vergu E, Boëlle PY. Flahault A, et al. BMC Infect Dis. 2009 Aug 12;9:129. doi: 10.1186/1471-2334-9-129. BMC Infect Dis. 2009. PMID: 19674455 Free PMC article. - Modeling the initial transmission dynamics of influenza A H1N1 in Guangdong Province, China.
Tan X, Yuan L, Zhou J, Zheng Y, Yang F. Tan X, et al. Int J Infect Dis. 2013 Jul;17(7):e479-84. doi: 10.1016/j.ijid.2012.11.018. Epub 2012 Dec 29. Int J Infect Dis. 2013. PMID: 23276487 - Influenza A (H1N1) 2009: a pandemic alarm.
Khanna M, Gupta N, Gupta A, Vijayan VK. Khanna M, et al. J Biosci. 2009 Sep;34(3):481-9. doi: 10.1007/s12038-009-0053-z. J Biosci. 2009. PMID: 19805908 Review. - [Swine-origin influenza H1N1/California--passions and facts].
Gendon IuZ. Gendon IuZ. Zh Mikrobiol Epidemiol Immunobiol. 2010 Jul-Aug;(4):105-14. Zh Mikrobiol Epidemiol Immunobiol. 2010. PMID: 20795393 Review. Russian.
Cited by
- Common trends in the epidemic of Covid-19 disease.
Radiom M, Berret JF. Radiom M, et al. Eur Phys J Plus. 2020;135(6):517. doi: 10.1140/epjp/s13360-020-00526-1. Epub 2020 Jun 22. Eur Phys J Plus. 2020. PMID: 32834912 Free PMC article. - A mathematical model for simulating the spread of a disease through a country divided into geographical regions with different population densities.
Harris PJ, Bodmann BEJ. Harris PJ, et al. J Math Biol. 2022 Sep 17;85(4):32. doi: 10.1007/s00285-022-01803-6. J Math Biol. 2022. PMID: 36114922 Free PMC article. - Are there medium to short-term multifaceted effects of the airborne pollutant PM2.5 determining the emergence of SARS-CoV-2 variants?
Baron YM. Baron YM. Med Hypotheses. 2021 Oct 20;158:110718. doi: 10.1016/j.mehy.2021.110718. Online ahead of print. Med Hypotheses. 2021. PMID: 34758423 Free PMC article. - Viral kinetics, stability and sensitivity analysis of the within-host COVID-19 model.
Elbaz IM, El-Metwally H, Sohaly MA. Elbaz IM, et al. Sci Rep. 2023 Jul 19;13(1):11675. doi: 10.1038/s41598-023-38705-6. Sci Rep. 2023. PMID: 37468601 Free PMC article. - An outbreak of influenza among trekkers in the Everest region of Nepal†.
Amatya B, Pandey P, Shrestha SK. Amatya B, et al. J Travel Med. 2020 Sep 26;27(6):taaa003. doi: 10.1093/jtm/taaa003. J Travel Med. 2020. PMID: 31943037 Free PMC article. No abstract available.
References
- Centers for Disease Control and Prevention http://www.cdc.gov/H1N1flu/
- Kermack WO, McKendrick AG. A contribution to the mathematical theory of epidemics. Proc Roy Soc Lond. 1927;115:700–721. doi: 10.1098/rspa.1927.0118. - DOI
- Rvachev LA. Modeling experiment of a large-scale epidemic by means of a computer. (In Russian.) Trans USSR Acad Sci Ser Mathematics and Physics. 1968;180:294–296.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials