Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer - PubMed (original) (raw)
Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer
Yao Dai et al. Int J Radiat Oncol Biol Phys. 2009.
Abstract
Purpose: Celastrol is an active ingredient of traditional herbal medicine and has recently been identified as a potent natural proteasome inhibitor. In the present study, we evaluated the radiosensitizing potential of celastrol in the human prostate cancer PC-3 model.
Methods and materials: Clonogenic assays were performed to determine the radiosensitizing effect of celastrol. Apoptosis was examined by flow cytometry using Annexin V and propidium iodide staining and by a caspase-3 activation assay. DNA damage processing was examined by immunofluorescent staining and Western blot for phosphorylated H2AX (gammaH2AX). The PC-3 xenograft model in the athymic nude mouse was used for the determination of the in vivo efficacy of celastrol combined with radiotherapy. The tumor samples were also analyzed for apoptosis and angiogenesis.
Results: Celastrol sensitized PC-3 cells to ionizing radiation (IR) in a dose- and schedule-dependent manner, in which pretreatment with celastrol for 1 h followed by IR achieved maximal radiosensitization. Celastrol significantly prolonged the presence of IR-induced gammaH2AX and increased IR-induced apoptosis. Celastrol, combined with fractionated radiation, significantly inhibited PC-3 tumor growth in vivo without obvious systemic toxicity. The combination treatment increased gammaH2AX levels and apoptosis, induced cleavage of poly(adenosine diphosphate-ribose)polymerase and Mcl-1, and reduced angiogenesis in vivo compared with either treatment alone.
Conclusion: Celastrol sensitized PC-3 cells to radiation both in vitro and in vivo by impairing DNA damage processing and augmenting apoptosis. Celastrol might represent a promising new adjuvant regimen for the treatment of hormone-refractory prostate cancer.
Conflict of interest statement
Conflict of Interest Notification: None
Figures
Fig. 1
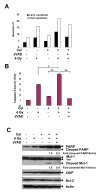
Enhanced effect of Celastrol (Cel) with ionizing radiation (IR) on PC-3 cells. A, Cel with IR showed enhanced cytotoxicity. Cells were pre-treated with the indicated concentrations of Cel for 1 h followed by IR. After irradiation, cells were incubated with Cel for 96 h and cell viability was measured. Bars, SD (_n_=3). *p<0.05; **p<0.01; ***p<0.001. B, Cel suppresses cell colony formation. Cells (150 cell/well) were treated with varying concentrations of Cel. Colonies (≥ 50 cells) were counted after 14 days’ incubation. Columns, mean; bars, SD (_n_=3). C, Cel sensitizes radiation-induced clonogenic cell death. Cells were pre-treated with the indicated concentrations of Cel for 1 h followed by irradiation. After irradiation, cells were incubated with Cel for another 24 h. Cells were then seeded into 6-well plates for clonogenic assay. Bars, SD (_n_=3). **p<0.01. Data represented one of three independent experiments. D, The schedule-dependence of Celastrol-mediated radiosensitization. Schedule I, cells were pretreated with 0.4 μM of Cel for 24 h then irradiated. Right after irradiation, cells were processed for clonogenic survival assay; Schedule II, cells were pretreated with 0.4 μM of Cel for 1 h followed by irradiation. After irradiation, cells were incubated with Cel for another 24 h before clonogenic survival assay; Schedule III, cells were pretreated with Cel for 24 h then irradiated. After irradiation, cells were incubated with Cel for another 24 h before clonogenic survival assay. Bars, SD (_n_=3). Data represent one of two independent experiments.
Fig. 2
Protein expression after treatment with Celastrol and IR. Cells were treated with Cel (0.4 μM) and IR (4 Gy) alone or in combination. For combination treatment, cells were exposed to irradiation and Cel following Schedule II in Fig. 1D. A, at desired time points after IR, cells were harvested and whole cell lysates were analyzed by Western blot. B, Relative expression of γH2AX in A was expressed by normalizing the ratio of the density to Actin in treated samples with untreated control. **p<0.01. Bars, SD of two independent experiments. C, Immunostaining of γH2AX by Celastrol and IR. Cells were fixed, permeablized, incubated with anti-γH2AX primary antibody and stained with FITC-labeled anti-mouse secondary antibody. Nuclear γH2AX foci were indicated as green fluorescent dots counterstained with DAPI for nuclear DNA (Original magnification, ×400). D, Quantification of γH2AX foci from C. Fifty cells were randomly selected for counting γH2AX foci (Original magnification, ×400). Columns, mean; bars, SE (_n_=50). ***p<0.001.
Fig. 3
Celastrol increased IR-induced apoptosis in PC-3 cells. Cells were treated with Cel (1 μM) and IR (4 Gy) alone or in combination for 72 h, with or without zVAD (2 μM) 1 h pretreatment. For combination treatment, cells were pre-treated with Cel for 1 h and exposed to IR. After irradiation, cells were incubated with Cel for 72 h. A, Cel enhanced IR-induced apoptosis. Cells were harvested and stained by Annexin V-FITC and PI, followed by analysis on flow cytometry. Both early [Annexin(+)/PI(−)] and total [Annexin(+)] apoptotic cell population were counted. Data shown represent one of two independent experiments. B, Cel enhanced IR-induced caspase-3 activation. Cells were lysed and incubate with fluorogenic substrate DEVD-AFC. Proteolytic release of AFC was monitored. Fold increase of fluorescent signal was calculated by dividing the normalized signal in each treated sample by that in the untreated control. Columns, mean; bars, SD (_n_=3). *p<0.05; **p<0.01. C, Combination of Cel with IR triggered PARP and Mcl-1 cleavage. Whole cell lysates (40 μg) were employed for Western blot. Relative expression of cleaved PARP and cleaved Mcl-1 was quantified by normalizing the ratio of the band intensity to Actin in treated samples with untreated control. *ns, non-specific band.
Fig. 4
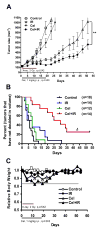
Antitumor effect of combination treatment in PC-3 xenografts. A, Nude mice bearing PC-3 tumors were treated as described in Materials and Methods. Tumor growth curve was plotted based on average tumor volumes. Bars, SE. **p<0.01 (two-way ANOVA). B, Kaplan-Meier curves show the effects of combining Cel with IR on tumor volume doubling time. The median tumor volume doubling time for each group is depicted numerically. Δ, p<0.0001 vs. IR (Mantel-Cox test). Tumor number (n) of each group is shown. C, Relative body weight was calculated by normalizing the average mice body weight in each group (8–10 mice/group) with the weight on the day of initial treatment (day 0).
Fig. 5
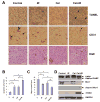
Immunohistological analysis and protein expression of tumor tissues. A, tumor sections on Day 18 were embedded in paraffin and processed to H&E, TUNEL, and anti-mouse CD31 immunostaining (original magnification, ×400). In TUNEL assay, the apoptotic cells were positively stained with brown nuclei (indicated by arrows). In CD31 assay, tumor blood vessels were stained brown (indicated by arrows). Both TUNEL-positive tumor cells (B) and anti-CD31-positive endothelial cells (C) were quantified by randomly counting 8 fields (original magnification, ×200). Columns, mean; bars, SEM (_n_=8). *p<0.05; **p<0.01. D, Whole cell lysates (30 μg) of tumor tissues was analyzed by Western blot with respective antibodies.
Similar articles
- Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB.
Dai Y, Desano J, Tang W, Meng X, Meng Y, Burstein E, Lawrence TS, Xu L. Dai Y, et al. PLoS One. 2010 Dec 10;5(12):e14153. doi: 10.1371/journal.pone.0014153. PLoS One. 2010. PMID: 21170316 Free PMC article. - Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis.
Dai Y, Liu M, Tang W, DeSano J, Burstein E, Davis M, Pienta K, Lawrence T, Xu L. Dai Y, et al. Clin Cancer Res. 2008 Dec 1;14(23):7701-10. doi: 10.1158/1078-0432.CCR-08-0188. Clin Cancer Res. 2008. PMID: 19047096 Free PMC article. - Radiosensitization of mammary carcinoma cells by telomere homolog oligonucleotide pretreatment.
Weng D, Cunin MC, Song B, Price BD, Eller MS, Gilchrest BA, Calderwood SK, Gong J. Weng D, et al. Breast Cancer Res. 2010;12(5):R71. doi: 10.1186/bcr2639. Epub 2010 Sep 16. Breast Cancer Res. 2010. PMID: 20846433 Free PMC article. - Radiosensitization in prostate cancer: mechanisms and targets.
Palacios DA, Miyake M, Rosser CJ. Palacios DA, et al. BMC Urol. 2013 Jan 26;13:4. doi: 10.1186/1471-2490-13-4. BMC Urol. 2013. PMID: 23351141 Free PMC article. Review.
Cited by
- Siah1 proteins enhance radiosensitivity of human breast cancer cells.
He HT, Fokas E, You A, Engenhart-Cabillic R, An HX. He HT, et al. BMC Cancer. 2010 Aug 3;10:403. doi: 10.1186/1471-2407-10-403. BMC Cancer. 2010. PMID: 20682032 Free PMC article. - Celastrol pretreatment as a therapeutic option against cisplatin-induced nephrotoxicity.
Boran T, Gunaydin A, Jannuzzi AT, Ozcagli E, Alpertunga B. Boran T, et al. Toxicol Res (Camb). 2019 Jul 31;8(5):723-730. doi: 10.1039/c9tx00141g. eCollection 2019 Sep 1. Toxicol Res (Camb). 2019. PMID: 31588349 Free PMC article. - The effect of celastrol in combination with 5-fluorouracil on proliferation and apoptosis of gastric cancer cell lines.
Moradi MT, Altememy D, Asadi-Samani M, Khosravian P, Soltani M, Hashemi L, Samiei-Sefat A. Moradi MT, et al. Oncol Res. 2024 Jun 20;32(7):1231-1237. doi: 10.32604/or.2024.047187. eCollection 2024. Oncol Res. 2024. PMID: 38948023 Free PMC article. - Celastrol and Its Role in Controlling Chronic Diseases.
Venkatesha SH, Moudgil KD. Venkatesha SH, et al. Adv Exp Med Biol. 2016;928:267-289. doi: 10.1007/978-3-319-41334-1_12. Adv Exp Med Biol. 2016. PMID: 27671821 Free PMC article. - Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy.
Chiu HW, Fang WH, Chen YL, Wu MD, Yuan GF, Ho SY, Wang YJ. Chiu HW, et al. PLoS One. 2012;7(7):e40462. doi: 10.1371/journal.pone.0040462. Epub 2012 Jul 3. PLoS One. 2012. PMID: 22802963 Free PMC article.
References
- Hanks GE, Hanlon AL, Hudes G, et al. Patterns-of-failure analysis of patients with high pretreatment prostate-specific antigen levels treated by radiation therapy: the need for improved systemic and locoregional treatment. J Clin Oncol. 1996;14:1093–1097. - PubMed
- Palayoor ST, Bump EA, Teicher BA, et al. Apoptosis and clonogenic cell death in PC3 human prostate cancer cells after treatment with gamma radiation and suramin. Radiation Research. 1997;148:105–114. - PubMed
- Oehler C, Dickinson DJ, Broggini-Tenzer A, et al. Current concepts for the combined treatment modality of ionizing radiation with anticancer agents. Curr Pharm Des. 2007;13:519–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA121830/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- 5 P30 CA46592/CA/NCI NIH HHS/United States
- R01 CA121830-01/CA/NCI NIH HHS/United States
- R21 CA128220-01/CA/NCI NIH HHS/United States
- R21 CA128220/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials