BDNF activates mTOR to regulate GluR1 expression required for memory formation - PubMed (original) (raw)
BDNF activates mTOR to regulate GluR1 expression required for memory formation
Leandro Slipczuk et al. PLoS One. 2009.
Abstract
Background: The mammalian target of Rapamycin (mTOR) kinase plays a key role in translational control of a subset of mRNAs through regulation of its initiation step. In neurons, mTOR is present at the synaptic region, where it modulates the activity-dependent expression of locally-translated proteins independently of mRNA synthesis. Indeed, mTOR is necessary for different forms of synaptic plasticity and long-term memory (LTM) formation. However, little is known about the time course of mTOR activation and the extracellular signals governing this process or the identity of the proteins whose translation is regulated by this kinase, during mnemonic processing.
Methodology/principal findings: Here we show that consolidation of inhibitory avoidance (IA) LTM entails mTOR activation in the dorsal hippocampus at the moment of and 3 h after training and is associated with a rapid and rapamycin-sensitive increase in AMPA receptor GluR1 subunit expression, which was also blocked by intra-hippocampal delivery of GluR1 antisense oligonucleotides (ASO). In addition, we found that pre- or post-training administration of function-blocking anti-BDNF antibodies into dorsal CA1 hampered IA LTM retention, abolished the learning-induced biphasic activation of mTOR and its readout, p70S6K and blocked GluR1 expression, indicating that BDNF is an upstream factor controlling mTOR signaling during fear-memory consolidation. Interestingly, BDNF ASO hindered LTM retention only when given into dorsal CA1 1 h after but not 2 h before training, suggesting that BDNF controls the biphasic requirement of mTOR during LTM consolidation through different mechanisms: an early one involving BDNF already available at the moment of training, and a late one, happening around 3 h post-training that needs de novo synthesis of this neurotrophin.
Conclusions/significance: IN CONCLUSION, OUR FINDINGS DEMONSTRATE THAT: 1) mTOR-mediated mRNA translation is required for memory consolidation during at least two restricted time windows; 2) this kinase acts downstream BDNF in the hippocampus and; 3) it controls the increase of synaptic GluR1 necessary for memory consolidation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
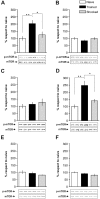
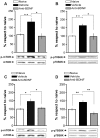
Figure 1. IA Training Is Associated with Two Time Windows of mTOR Activation in the Hippocampus.
Bars represent the mean p-mTOR/mTOR ratio of trained (black) or shocked (gray) groups respect to naïve (white) group, sacrificed immediately (A), 15 min (B), 1 h (C), 3 h (D), 9 h (E) or 12 h (F) after IA training. Data are expressed as means±SEM of p-mTOR/mTOR ratio; *p<0.05, **p<0.01. Representative blots of phosphorylated and total protein levels of mTOR are shown in the lower panels. n = 5–6 per group for each experiment.
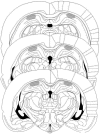
Figure 2. Cannulae Placements and Drug Infusions.
Schematic representations of rat brain sections at three rostrocaudal planes (−3.80, −4.30, and −4.80 from bregma) taken from the atlas of Paxinos and Watson, showing, in stippling, the extension of the area reached by the infusions in the dorsal hippocampus. Reprinted from The Rat Brain in Stereotaxic Coordinates by Paxinos and Watson, pages 33, 35, and 37, Academic Press (1997), with permission from Elsevier.
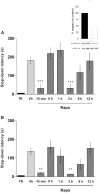
Figure 3. mTOR Signaling Is Required for IA Memory Consolidation During Two Restricted Time Windows After Training.
Data are expressed as mean (±SEM) of training (TR, black bars) or test session step-down latency at 24 h (A) or 7 days (B) after IA training. Animals were infused intra-CA1 of the dorsal hippocampus with vehicle (Vh, light grey bars) or rapamycin (4.3 pg/side, dark grey bars), 15 min before or 0, 1, 3, 9, and 12 h after training. Inlet: Rats were infused into the dorsal hippocampus with rapamycin (4.3 pg/side) or vehicle and sacrificed 15 min after drug infusion for analysis of p70S6K activation in the dorsal hippocampus by Western blot. Bars represent the p-p70S6K/p70S6K ratio of rapamycin (white) respect to vehicle (black) treated rats. *p<0.05, **p<0.01, ***p<0.001 vs. Vh for each time point, n = 8–10 per group for each experiment in A and B and n = 5 per group in the inlet.
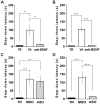
Figure 4. Different Requirement of BDNF Activity and Synthesis During the Two Time Windows of Memory Consolidation.
Data are expressed as mean (±SEM) of training (TR, black bars) or test session step-down latency at 24 h after IA training. Animals were infused intra-CA1 of the dorsal hippocampus with vehicle (Vh, white bars) or function-blocking anti-BDNF antibodies (1 µg/µl, grey bars) 15 min before (A) or 3 h (B) after training. Animals were infused intra-CA1 of the dorsal hippocampus with BDNF MSO (white bar) or BDNF ASO (2 nmol/µl, 1 µg/side, grey bar), 2 h before (C) or 1 h (D) after IA training. *p<0.05, **p<0.01, ***p<0.001 vs Vh (A, B) or MSO (C, D), n = 10 per group for each experiment.
Figure 5. BDNF Triggers mTOR Activation in the Hippocampus During IA Training and 3 h Thereafter.
Bars represent mean p-mTOR/mTOR (A and C) or p-p70S6K/p70S6K ratio (B and D) of rats infused with vehicle (black), function-blocking anti-BDNF antibodies (0.5 µg/side; grey), respect to the naïve group (white). Intra-CA1 infusion of function-blocking anti-BDNF antibodies 15 min before IA training prevents mTOR (A) and p70S6K (B) activation immediately or 15 min after training respectively and infusion 2∶45 h after training prevents mTOR (C) and p70S6K (D) activation 3 h after training. Representative blots of phosphorylated and total protein levels of mTOR and p70S6K are shown in the lower panels. *p<0.05, **p<0.01, n = 5–6 per group for each experiment.
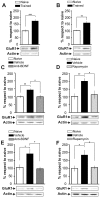
Figure 6. IA Training Induces GluR1 Expression in Hippocampal Synaptic Plasma Membranes-Enriched Fractions Through BDNF/mTOR Pathway.
Bars represent the mean GluR1/actin ratio from synaptic plasma membranes-enriched fractions obtained from samples of the dorsal hippocampus of trained (black) respect to naïve (white) rats 15 min (A) or 3 h after IA training (B). Rats infused intra-CA1 of the dorsal hippocampus 15 min before and sacrificed 15 min after training (B and C) or 2∶45 h and sacrificed 3 h both after training (E and F) with vehicle (black) or function-blocking anti-BDNF antibodies (0.5 µg/side; dark grey) (C and E) or rapamycin (4.3 pg/side; light grey) (D and F). Representative blots of total GluR1 protein levels and actin are shown in the lower panels *p<0.05, **p<0.01, ***p<0.001, n = 5–7 per group.
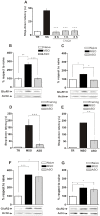
Figure 7. GluR1 Translation Is Required for IA Memory Consolidation During Training and 3 h Thereafter.
(A) Animals were injected with Vehicle (black bar) or CNQX immediately, 1 h or 3 h after IA training (grey bars). Data are expressed as mean (±SEM) of training (TR, white bars) or test session step-down latency at 24 h after IA training. (B and C) Bars represent the mean GluR1/actin ratio from synaptic plasma membranes-enriched fractions obtained from samples of the dorsal hippocampus of animals trained in IA and injected with GluR1 ASO 2 h pre-TR (grey bar) or MSO (black bar) and sacrificed 15 min after TR (B) or injected with GluR1 ASO 1 h after TR or MSO and sacrificed 3 h after TR (C). (D and E) Animals were injected with MSO (black bar) or GluR1 ASO (grey bar) 2 h pre-TR (D) or 1 h after TR (E). Data are expressed as mean (±SEM) of training (TR, white bars) or test session step-down latency at 24 h after IA training. (F and G) Bars represent the mean GluR2/actin ratio from synaptic plasma membranes-enriched fractions obtained from samples of the dorsal hippocampus of animals trained in IA and injected with GluR1 ASO 2 h pre-TR (grey bar) or MSO (black bar) and sacrificed 15 min after TR (B) or injected with GluR1 ASO 1 h after TR or MSO and sacrificed 3 h after TR (C). *p<0.05, **p<0.01, ***p<0.001, n = 10–14 per group for each experiment in figures A, D and E; n = 5 per group for each experiment in figures B, C, F and G.
Similar articles
- BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation.
Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. Alonso M, et al. Hippocampus. 2002;12(4):551-60. doi: 10.1002/hipo.10035. Hippocampus. 2002. PMID: 12201640 - mTOR signaling in the hippocampus is necessary for memory formation.
Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. Bekinschtein P, et al. Neurobiol Learn Mem. 2007 Feb;87(2):303-7. doi: 10.1016/j.nlm.2006.08.007. Epub 2006 Sep 26. Neurobiol Learn Mem. 2007. PMID: 17005423 - Beta-adrenergic receptors link NO/sGC/PKG signaling to BDNF expression during the consolidation of object recognition long-term memory.
Furini CR, Rossato JI, Bitencourt LL, Medina JH, Izquierdo I, Cammarota M. Furini CR, et al. Hippocampus. 2010 May;20(5):672-83. doi: 10.1002/hipo.20656. Hippocampus. 2010. PMID: 19533679 - BDNF-induced local protein synthesis and synaptic plasticity.
Leal G, Comprido D, Duarte CB. Leal G, et al. Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review. - The integrated role of ACh, ERK and mTOR in the mechanisms of hippocampal inhibitory avoidance memory.
Giovannini MG, Lana D, Pepeu G. Giovannini MG, et al. Neurobiol Learn Mem. 2015 Mar;119:18-33. doi: 10.1016/j.nlm.2014.12.014. Epub 2015 Jan 13. Neurobiol Learn Mem. 2015. PMID: 25595880 Review.
Cited by
- Arc and BDNF mediated effects of hippocampal astrocytic glutamate uptake blockade on spatial memory stages.
Riboldi JG, Correa J, Renfijes MM, Tintorelli R, Viola H. Riboldi JG, et al. Commun Biol. 2024 Aug 22;7(1):1032. doi: 10.1038/s42003-024-06586-8. Commun Biol. 2024. PMID: 39174690 Free PMC article. - BDNF/TrkB signalling, in cooperation with muscarinic signalling, retrogradely regulates PKA pathway to phosphorylate SNAP-25 and Synapsin-1 at the neuromuscular junction.
Polishchuk A, Cilleros-Mañé V, Balanyà-Segura M, Just-Borràs L, Forniés-Mariné A, Silvera-Simón C, Tomàs M, Jami El Hirchi M, Hurtado E, Tomàs J, Lanuza MA. Polishchuk A, et al. Cell Commun Signal. 2024 Jul 23;22(1):371. doi: 10.1186/s12964-024-01735-2. Cell Commun Signal. 2024. PMID: 39044222 Free PMC article. - Rapamycin attenuates reconsolidation of a backwards-conditioned aversive stimuli in female mice.
Trask J, MacCallum PE, Rideout H, Preisser EL, Blundell JJ. Trask J, et al. Psychopharmacology (Berl). 2024 Mar;241(3):601-612. doi: 10.1007/s00213-024-06544-6. Epub 2024 Feb 5. Psychopharmacology (Berl). 2024. PMID: 38311691 - The Possible Role of Brain-derived Neurotrophic Factor in Epilepsy.
AlRuwaili R, Al-Kuraishy HM, Al-Gareeb AI, Ali NH, Alexiou A, Papadakis M, Saad HM, Batiha GE. AlRuwaili R, et al. Neurochem Res. 2024 Mar;49(3):533-547. doi: 10.1007/s11064-023-04064-x. Epub 2023 Nov 25. Neurochem Res. 2024. PMID: 38006577 Free PMC article. Review. - The Multiple Roles of Autophagy in Neural Function and Diseases.
Li YY, Qin ZH, Sheng R. Li YY, et al. Neurosci Bull. 2024 Mar;40(3):363-382. doi: 10.1007/s12264-023-01120-y. Epub 2023 Oct 19. Neurosci Bull. 2024. PMID: 37856037 Free PMC article. Review.
References
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. - PubMed
- McGaugh JL. Memory–a century of consolidation. Science. 2000;287:248–251. - PubMed
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. - PubMed
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous