Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype - PubMed (original) (raw)
Comparative Study
Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype
Gilles Vandewalle et al. J Neurosci. 2009.
Abstract
Cognition is regulated across the 24 h sleep-wake cycle by circadian rhythmicity and sleep homeostasis through unknown brain mechanisms. We investigated these mechanisms in a functional magnetic resonance imaging study of executive function using a working memory 3-back task during a normal sleep-wake cycle and during sleep loss. The study population was stratified according to homozygosity for a variable-number (4 or 5) tandem-repeat polymorphism in the coding region of the clock gene PERIOD3. This polymorphism confers vulnerability to sleep loss and circadian misalignment through its effects on sleep homeostasis. In the less-vulnerable genotype, no changes were observed in brain responses during the normal-sleep wake cycle. During sleep loss, these individuals recruited supplemental anterior frontal, temporal and subcortical regions, while executive function was maintained. In contrast, in the vulnerable genotype, activation in a posterior prefrontal area was already reduced when comparing the evening to the morning during a normal sleep-wake cycle. Furthermore, in the morning after a night of sleep loss, widespread reductions in activation in prefrontal, temporal, parietal and occipital areas were observed in this genotype. These differences occurred in the absence of genotype-dependent differences in circadian phase. The data show that dynamic changes in brain responses to an executive task evolve across the sleep-wake and circadian cycles in a regionally specific manner that is determined by a polymorphism which affects sleep homeostasis. The findings support a model of individual differences in executive control, in which the allocation of prefrontal resources is constrained by sleep pressure and circadian phase.
Figures
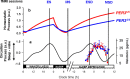
Figure 1.
Schematic representation of the protocol and the homeostatic and circadian processes in PER34/4 and PER35/5. All times are in clock time (h). a, Circadian arousal signal (Sleep-Wake) [arbitrary units (a.u.)], which promotes wakefulness during the day and sleep at night (based on data published in Dijk et al., 1997) oscillates independently from sleep-wake behavior and similarly in PER34/4 and PER3 5/5. The nadir of the circadian arousal rhythm is located in the early morning and its crest at the end of the habitual waking day. The time course of melatonin during the sleep deprivation condition (means + SE; SEs are not plotted for n ≤2) is plotted to the right. b, Homeostatic sleep pressure (a.u.) increases during wakefulness, declines during sleep, and increases further during sleep deprivation. Based on waking and sleep EEG data (Viola et al., 2007), homeostatic sleep pressure increases and declines more rapidly in PER35/5. Vertical lines, Positions of the different fMRI sessions.
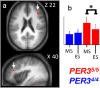
Figure 2.
Significant differences in brain response between the sessions recorded after 14 h and 1.5 h of wakefulness. a, PER35/5 significantly reduced response in the right posterior inferior frontal gyrus during ES compared with MS. Statistical results are overlaid to the population mean structural image (p uncorrected < 0.001). No significant changes were observed in PER34/4. b, Mean activity estimates (a.u. ± SEM; in all figures * indicates the significant differences at p < 0.05 at the voxel level after correction for multiple comparisons).
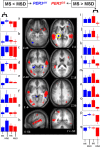
Figure 3.
Significant differences in brain response between the sessions recorded after 25 h and 1.5 h of wakefulness. Middle panels, In PER34/4 (blue), only significant increased activations were found in MSD compared with MS. In PER35/5 (red), only significant reduced activations were found in MSD compared with MS (see Table 2 for the names corresponding to the letters in the structural image). The difference in overnight change in activity in the area circled in yellow (thalamus; Z 4 brain slice) on the nights with and without sleep was significantly and negatively correlated with the self-assessed likelihood to fall asleep in nonstimulating situations during a normal waking day. Lateral panels, Mean activity estimates (a.u. ± SEM). Note that in the left panels (a–h), for all areas indicated by an asterisk (*), there were significant increases in activation, i.e., only in PER34/4, whereas in the right panels (i–q) for all areas indicated by an asterisk (*), there were decreases in activation, i.e., only in PER35/5. Note that in the middle frontal gyrus (i), difference is also significant between MS and ES as reported in Figure 2.
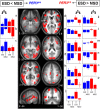
Figure 4.
Significant differences in brain response between the sessions recorded after 25 and 14 h of wakefulness. Middle panels, In PER34/4 (blue), only significant increased activations were found in MSD compared with ESD. In PER35/5 (red), only significant reduced activations were found in MSD compared with ESD (see Table 3 for the names corresponding to the letters in the structural image). Lateral panels, Mean activity estimates (a.u. ± SEM) at ESD and MSD. Left (a–c) ESD < MSD; right (**_d–p_**) ESD > MSD. Note that in left panels, for all areas indicated by an asterisk (*), there were significant increases in activation, i.e., only in PER34/4, whereas in right panels, for all areas indicated by an asterisk (*), there were significant decreases in activation, i.e., only in PER35/5.
Similar articles
- PERIOD3, circadian phenotypes, and sleep homeostasis.
Dijk DJ, Archer SN. Dijk DJ, et al. Sleep Med Rev. 2010 Jun;14(3):151-60. doi: 10.1016/j.smrv.2009.07.002. Epub 2009 Aug 29. Sleep Med Rev. 2010. PMID: 19716732 Review. - Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism.
Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Groeger JA, et al. Sleep. 2008 Aug;31(8):1159-67. Sleep. 2008. PMID: 18714788 Free PMC article. - PER3 polymorphism predicts sleep structure and waking performance.
Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, von Schantz M, Dijk DJ. Viola AU, et al. Curr Biol. 2007 Apr 3;17(7):613-8. doi: 10.1016/j.cub.2007.01.073. Epub 2007 Mar 8. Curr Biol. 2007. PMID: 17346965 - PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase.
Viola AU, James LM, Archer SN, Dijk DJ. Viola AU, et al. Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2156-63. doi: 10.1152/ajpheart.00662.2008. Epub 2008 Oct 3. Am J Physiol Heart Circ Physiol. 2008. PMID: 18835917 Free PMC article. - Circadian clock genes and sleep homeostasis.
Franken P, Dijk DJ. Franken P, et al. Eur J Neurosci. 2009 May;29(9):1820-9. doi: 10.1111/j.1460-9568.2009.06723.x. Epub 2009 Apr 28. Eur J Neurosci. 2009. PMID: 19473235 Review.
Cited by
- A Topological Cluster of Differentially Regulated Genes in Mice Lacking PER3.
Van der Veen DR, Laing EE, Bae SE, Johnston JD, Dijk DJ, Archer SN. Van der Veen DR, et al. Front Mol Neurosci. 2020 Feb 13;13:15. doi: 10.3389/fnmol.2020.00015. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32116548 Free PMC article. - The sleep-deprived human brain.
Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, Walker MP. Krause AJ, et al. Nat Rev Neurosci. 2017 Jul;18(7):404-418. doi: 10.1038/nrn.2017.55. Epub 2017 May 18. Nat Rev Neurosci. 2017. PMID: 28515433 Free PMC article. Review. - Sustained wakefulness and visual attention: moderation by chronotype.
Barclay NL, Myachykov A. Barclay NL, et al. Exp Brain Res. 2017 Jan;235(1):57-68. doi: 10.1007/s00221-016-4772-8. Epub 2016 Sep 13. Exp Brain Res. 2017. PMID: 27624836 Free PMC article. - Functional neuroimaging of the reciprocal influences between sleep and wakefulness.
Jedidi Z, Rikir E, Muto V, Mascetti L, Kussé C, Foret A, Shaffii-Le Bourdiec A, Vandewalle G, Maquet P. Jedidi Z, et al. Pflugers Arch. 2012 Jan;463(1):103-9. doi: 10.1007/s00424-011-1016-4. Epub 2011 Sep 16. Pflugers Arch. 2012. PMID: 21922188 Review. - Neuroimaging, cognition, light and circadian rhythms.
Gaggioni G, Maquet P, Schmidt C, Dijk DJ, Vandewalle G. Gaggioni G, et al. Front Syst Neurosci. 2014 Jul 8;8:126. doi: 10.3389/fnsys.2014.00126. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25071478 Free PMC article. Review.
References
- Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. - PubMed
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. - PubMed
- Aston-Jones G. Brain structures and receptors involved in alertness. Sleep Med. 2005;6(Suppl 1):S3–S7. - PubMed
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical