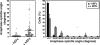Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia - PubMed (original) (raw)
Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia
Nicole den Elzen et al. Mol Biol Cell. 2009 Aug.
Abstract
Oriented cell division is a fundamental determinant of tissue organization. Simple epithelia divide symmetrically in the plane of the monolayer to preserve organ structure during epithelial morphogenesis and tissue turnover. For this to occur, mitotic spindles must be stringently oriented in the Z-axis, thereby establishing the perpendicular division plane between daughter cells. Spatial cues are thought to play important roles in spindle orientation, notably during asymmetric cell division. The molecular nature of the cortical cues that guide the spindle during symmetric cell division, however, is poorly understood. Here we show directly for the first time that cadherin adhesion receptors are required for planar spindle orientation in mammalian epithelia. Importantly, spindle orientation was disrupted without affecting tissue cohesion or epithelial polarity. This suggests that cadherin receptors can serve as cues for spindle orientation during symmetric cell division. We further show that disrupting cadherin function perturbed the cortical localization of APC, a microtubule-interacting protein that was required for planar spindle orientation. Together, these findings establish a novel morphogenetic function for cadherin adhesion receptors to guide spindle orientation during symmetric cell division.
Figures
Figure 1.
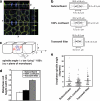
Spindle orientation in the plane of the monolayer does not correlate with changes in cell geometry. (a) XYZ projections of a confocal Z-stack taken of an MDCK monolayer stained for β-catenin (green), γ-tubulin (red), and DNA (blue), showing an anaphase cell dividing symmetrically. Apical is up. White lines indicate the planes at which orthogonal views were taken. Scale bar, 5 μm. (b) Schematic diagram showing metaphase cell dimensions and spindle lengths (pole-to-pole) from MDCK monolayers cultured on coverslips and grown to subconfluence or 100% confluence or grown to high density on transwell filters in order to obtain different cell geometries (mean ± SE, n ≥ 50 cells, pooled from three independent experiments). Cell dimensions were taken in metaphase, as this is when the spindle is at its longest before sister chromatid separation, and thus when any geometric constraints on spindle movements and spindle orientation would be expected to be at their greatest. (c) Diagram of how spindle angles were calculated from confocal Z-stacks. Spindles oriented parallel to the plane of the monolayer had an angle of 0°, whereas spindles perpendicular to the monolayer had an angle of 90°. (d) Metaphase cell height-width ratios from the cells in b (mean ± SE). Cell height-width ratios increased with cell confluency. (e) Spindle angles relative to the plane of the monolayer of anaphase cells from the same experiments as in b and c. In each case, the spread and overall mean of anaphase spindle angles are shown (n ≥ 50 cells). Anaphase cells were used for spindle angle measurements to avoid inaccuracies resulting from spindle orientation changes during metaphase. No correlation was found between anaphase spindle angle and cell height-width ratios (two-tailed Spearman correlation test; α = 0.05; r = 0.50, p = 1.0).
Figure 2.
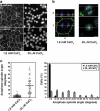
Spindles misorient when cell–cell contacts are disrupted. (a) Localization of E-cadherin and β-catenin in MDCK cells incubated in physiological (1.8 mM) or 30 μM CaCl2 for 1.5 h to disrupt cell–cell contacts. Bar, 20 μm. (b) XY and XZ projections of confocal Z-stacks taken of anaphase cells stained for β-catenin (green), γ-tubulin (red), and DNA (blue). The anaphase cell in 30 μM CaCl2 has not divided within the plane of the monolayer, in contrast to the cell in 1.8 mM CaCl2. In this, and subsequent figures, apical is up and white lines indicate the planes at which orthogonal views were taken. Bar, 5 μm. (c) Anaphase spindle angles of cells incubated in 1.8 mM or 30 μM CaCl2. For each condition, the spread and overall mean of spindle angles from three independent experiments, each with n ≥ 30 cells, together with a frequency distribution of spindle angles (mean ± SE of the three experiments), are shown. Spindle orientation in the plane of the monolayer was perturbed by calcium depletion (two-tailed Mann-Whitney test, p < 0.0001).
Figure 3.
Spindles misorient when E-cadherin ligation is blocked. (a) E-cadherin and β-catenin staining in MCF10A cells incubated in physiological CaCl2 (1.8 mM), in the absence of CaCl2 for 2 h or in the presence or absence of E-cadherin antibody SHE78-7 for 5 h when CaCl2 was restored after depletion to disrupt cell–cell contacts. Bar, 20 μm. (b) XY and XZ projections of anaphase cells stained for β-catenin (green), γ-tubulin (red), and DNA (blue). Anaphase cells from monolayers treated with 0 mM CaCl2 or E-cadherin–blocking antibody have not divided within the plane of the monolayer, whereas control anaphase cells have. Bar, 5 μm. (c) Anaphase spindle angles of cells treated as in panel a. Data represented as in Figure 2. Both calcium depletion and E-cadherin blocking antibody affected spindle orientation (two-tailed Mann-Whitney tests, p < 0.0001).
Figure 4.
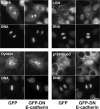
Cadherin acts as a spatial cue to orient the mitotic spindle independently of cell–cell cohesion. (a) Schematic diagram of a dominant-negative mutant of human E-cadherin. (b) Immunofluorescence characterization of E-cadherin and catenins in MDCK clones stably expressing GFP-DN E-cadherin or GFP protein alone. (c) Protein expression in MDCK clones stably expressing GFP-DN E-cadherin or GFP protein alone. Western blots of cell lysates were probed for GFP (identifying the transgenes), E-cadherin (identifying both endogenous protein [single arrow], and the mutant transgene [double arrow]), β-catenin, and α-catenin. GAPDH or β-tubulin were used as loading controls. (d) XY and XZ projections of anaphase cells stained for β-catenin (green), γ-tubulin (red), and DNA (blue). The anaphase cell expressing GFP-DN E-cadherin has not divided within the plane of the monolayer (XY); note also that the x-y confocal slice is taken high in the dividing cell, so that surrounding cells in the monolayer are not apparent. Bars, 5 μm. (e) Anaphase spindle angles of MDCK clones expressing GFP-DN E-cadherin or GFP. Data are represented as in Figure 2. For both GFP-DN E-cadherin and GFP, data from two clones, each of which gave similar results, were pooled. Spindle orientation was affected by expression of GFP-DN E-cadherin (two-tailed Mann-Whitney test, p < 0.0001).
Figure 5.
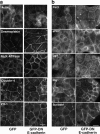
Dominant-negative cadherin does not disrupt cell–cell junctions or polarity determinants. Confluent MDCK monolayers stably expressing GFP-DN E-cadherin or GFP alone were examined by immunofluorescence microscopy. All experiments were performed under identical culture conditions and confluency as the experiments measuring spindle orientation. Two clones were analyzed for each construct, both of which gave comparable results. Scale bar, 20 μm. Monolayers were stained for junctional markers (a) or polarity determinants (b), as indicated.
Figure 6.
Localized cadherin homophilic ligation alters spindle orientation in nonpolarized cells. Anaphase spindle angles relative to the plane of the substratum of isolated hE-CHO cells plated for 1.5 h onto hE/Fc-coated or onto control coverslips (data pooled from three independent experiments, each with n ≥ 13 cells). Plating cells onto E-cadherin ligand altered spindle orientation (two-tailed Mann-Whitney test, p < 0.0001).
Figure 7.
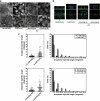
E-cadherin is sufficient, but not essential, to control spindle orientation in MDCK cell monolayers. (a) Western blots showing siRNA depletion of E-cadherin and cadherin-6 in MDCK cells and in MDCK cells stably expressing a human E-cadherin-GFP construct resistant to the siRNAs. GAPDH was used as a loading control. Arrowhead indicates human E-cadherin-GFP. (b) F-actin immunofluorescence of E-cadherin KD MDCK cells, double KD MDCK cells and double KD cells expressing human E-Cadherin-GFP. Fields of cells without knockdown are shown adjacent to KD cells for comparison. Bars, 20 μm. (c) XY and XZ projections of anaphase cells stained for β-catenin (green), γ-tubulin (red), and DNA (blue). Control, E-cadherin KD and human E-cadherin-GFP–expressing double KD cells have all divided within the plane of the monolayer, whereas the double KD MDCK cell has not. Bar, 5 μm. (d) Anaphase spindle angles of cells treated as in panel c. Data are represented as in Figure 2. Spindle orientation was not affected by E-cadherin KD alone (two-tailed Mann-Whitney test, p >0.05), but was affected by KD of both E-cadherin and cadherin-6 (p < 0.0001). Correct spindle orientation in double KD cells was restored by expression of human E-cadherin-GFP (p > 0.05).
Figure 8.
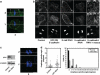
Cortical localization patterns of NuMA, LGN, dynein, and p150Glued in mitotic MDCK cells expressing GFP-DN E-cadherin or GFP. Cortical localization was not overtly perturbed by GFP-DN E-cadherin expression. All experiments were performed under identical culture conditions and confluency as the experiments measuring spindle orientation. Scale bar, 10 μm.
Figure 9.
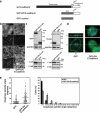
Loss of junctional APC correlates with conditions that cause spindle misorientation. (a) XY and XZ projections of a mitotic MDCK cell stained for APC (green), γ-tubulin (red), and DNA (blue). APC localized to the cortex in the same Z-region as the spindle poles. Arrowheads indicate apicolateral cortical staining of APC. Bar, 5 μm. (b) Localization of APC in MDCK cells treated with GFP-DN E-cadherin, E-cadherin RNAi, combined E-cadherin/cadherin-6 RNAi and double KD cells expressing human E-cadherin-GFP. For the rescue experiment, costaining for human E-cadherin-GFP is shown. In all other cases, costaining for β-catenin, whose loss from cell junctions also correlated with conditions that caused spindle misorientation, is shown. Bar, 5 μm. (c) Depletion of cellular APC by siRNA. APC levels following siRNA transfection were assessed by Western analysis in cell lysates and by immunofluorescence in mitotic cells. XY and XZ projections of anaphase cells stained for APC (green), γ-tubulin (red), and DNA (blue). Note loss of junctional APC staining in APC RNAi cells. (d) Anaphase spindle angles of control and APC RNAi MDCK cells. Data represented as in Figure 2 and pooled from three independent experiments (each with n ≥ 30). Spindle orientation was affected by APC RNAi (two-tailed Mann-Whitney test, p < 0.0001).
Similar articles
- Myosin 2-Induced Mitotic Rounding Enables Columnar Epithelial Cells to Interpret Cortical Spindle Positioning Cues.
Chanet S, Sharan R, Khan Z, Martin AC. Chanet S, et al. Curr Biol. 2017 Nov 6;27(21):3350-3358.e3. doi: 10.1016/j.cub.2017.09.039. Epub 2017 Oct 26. Curr Biol. 2017. PMID: 29107549 - Cell shape and intercellular adhesion regulate mitotic spindle orientation.
Li J, Cheng L, Jiang H. Li J, et al. Mol Biol Cell. 2019 Sep 1;30(19):2458-2468. doi: 10.1091/mbc.E19-04-0227. Epub 2019 Aug 14. Mol Biol Cell. 2019. PMID: 31411941 Free PMC article. - E-cadherin and LGN align epithelial cell divisions with tissue tension independently of cell shape.
Hart KC, Tan J, Siemers KA, Sim JY, Pruitt BL, Nelson WJ, Gloerich M. Hart KC, et al. Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):E5845-E5853. doi: 10.1073/pnas.1701703114. Epub 2017 Jul 3. Proc Natl Acad Sci U S A. 2017. PMID: 28674014 Free PMC article. - Epithelial polarity and spindle orientation: intersecting pathways.
Bergstralh DT, Haack T, St Johnston D. Bergstralh DT, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Sep 23;368(1629):20130291. doi: 10.1098/rstb.2013.0291. Print 2013. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24062590 Free PMC article. Review. - Cell adhesion molecule control of planar spindle orientation.
Tuncay H, Ebnet K. Tuncay H, et al. Cell Mol Life Sci. 2016 Mar;73(6):1195-207. doi: 10.1007/s00018-015-2116-7. Epub 2015 Dec 23. Cell Mol Life Sci. 2016. PMID: 26698907 Free PMC article. Review.
Cited by
- Syntaxin 16 regulates lumen formation during epithelial morphogenesis.
Jung JJ, Inamdar SM, Tiwari A, Ye D, Lin F, Choudhury A. Jung JJ, et al. PLoS One. 2013 Apr 23;8(4):e61857. doi: 10.1371/journal.pone.0061857. Print 2013. PLoS One. 2013. PMID: 23626741 Free PMC article. - Random chromosome segregation in mouse intestinal epithelial stem cells.
Legraverend C, Jay P. Legraverend C, et al. Chromosome Res. 2013 May;21(3):213-24. doi: 10.1007/s10577-013-9351-2. Chromosome Res. 2013. PMID: 23681655 Review. - The LKB1 tumor suppressor controls spindle orientation and localization of activated AMPK in mitotic epithelial cells.
Wei C, Bhattaram VK, Igwe JC, Fleming E, Tirnauer JS. Wei C, et al. PLoS One. 2012;7(7):e41118. doi: 10.1371/journal.pone.0041118. Epub 2012 Jul 18. PLoS One. 2012. PMID: 22815934 Free PMC article. - Zebrafish neural tube morphogenesis requires Scribble-dependent oriented cell divisions.
Žigman M, Trinh le A, Fraser SE, Moens CB. Žigman M, et al. Curr Biol. 2011 Jan 11;21(1):79-86. doi: 10.1016/j.cub.2010.12.005. Epub 2010 Dec 23. Curr Biol. 2011. PMID: 21185191 Free PMC article. - Cadherins and Pak1 control contact inhibition of proliferation by Pak1-betaPIX-GIT complex-dependent regulation of cell-matrix signaling.
Liu F, Jia L, Thompson-Baine AM, Puglise JM, Ter Beest MB, Zegers MM. Liu F, et al. Mol Cell Biol. 2010 Apr;30(8):1971-83. doi: 10.1128/MCB.01247-09. Epub 2010 Feb 12. Mol Cell Biol. 2010. PMID: 20154149 Free PMC article.
References
- Ahringer J. Control of cell polarity and mitotic spindle positioning in animal cells. Curr. Opin. Cell Biol. 2003;15:73–81. - PubMed
- Baena-Lopez L. A., Baonza A., Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 2005;15:1640–1644. - PubMed
- Betschinger J., Knoblich J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 2004;14:R674–685. - PubMed
- Busson S., Dujardin D., Moreau A., Dompierre J., De Mey J. R. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 1998;8:541–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous