PR55 alpha, a regulatory subunit of PP2A, specifically regulates PP2A-mediated beta-catenin dephosphorylation - PubMed (original) (raw)
PR55 alpha, a regulatory subunit of PP2A, specifically regulates PP2A-mediated beta-catenin dephosphorylation
Wen Zhang et al. J Biol Chem. 2009.
Abstract
A central question in Wnt signaling is the regulation of beta-catenin phosphorylation and degradation. Multiple kinases, including CKI alpha and GSK3, are involved in beta-catenin phosphorylation. Protein phosphatases such as PP2A and PP1 have been implicated in the regulation of beta-catenin. However, which phosphatase dephosphorylates beta-catenin in vivo and how the specificity of beta-catenin dephosphorylation is regulated are not clear. In this study, we show that PP2A regulates beta-catenin phosphorylation and degradation in vivo. We demonstrate that PP2A is required for Wnt/beta-catenin signaling in Drosophila. Moreover, we have identified PR55 alpha as the regulatory subunit of PP2A that controls beta-catenin phosphorylation and degradation. PR55 alpha, but not the catalytic subunit, PP2Ac, directly interacts with beta-catenin. RNA interference knockdown of PR55 alpha elevates beta-catenin phosphorylation and decreases Wnt signaling, whereas overexpressing PR55 alpha enhances Wnt signaling. Taken together, our results suggest that PR55 alpha specifically regulates PP2A-mediated beta-catenin dephosphorylation and plays an essential role in Wnt signaling.
Figures
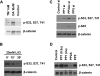
FIGURE 1.
Screening of phosphatases that regulate β-catenin phosphorylation. A, HEK293T cells were treated with 1 n
m
tautomycine (TM), 10 n
m
okadaic acid (OA), and dimethyl sulfoxide (Control), respectively. Cytoplasmic fractions were isolated and analyzed by Western blot using Abs that recognize total β-catenin or GSK3-phosphorylated β-catenin (p-S33, S37, T41). B, SW480 cells were treated with 25 m
m
LiCl for 0, 15, and 30 min. Cell lysates were analyzed by Western blot using Abs that recognize total β-catenin or phosphorylated β-catenin. C, SW480 cell were transfected with control siRNA and siRNAs for the PP1, PP5, and the catalytic subunit of PP2A (PP2Ac). Cell lysates were analyzed by Western blot using Abs that recognize total β-catenin, CKIα-phosphorylated β-catenin (p-S45), or GSK3-phosphorylated β-catenin. D, phosphorylated β-catenin was immunoprecipitated from HCT116 cells. Equal amounts of β-catenin were incubated with purified PP1 (0.5 and 2 units), PP2A (0.5 unit), and PP5 for 30 min. Phosphatase buffer alone was used as a control. β-Catenin dephosphorylation was analyzed by Western blot using Abs that recognize total β-catenin or phosphorylated β-catenin.
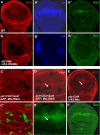
FIGURE 2.
PP2A is essential for Drosophila Wg signaling. A, _A_′, and _A_″, a wild-type wing disc was stained with anti-Arm, Dll, and Sen. B, _B_′, and _B_″, UAS-DN-mts was expressed by MS1096 gal4 and immunostained for Arm, Dll, and Sen. C and _C_′, UAS-MtsRNAi was expressed by _act_>_CD2_>gal4 and larvae were grown at 20 °C, limiting the strength of mts RNAi that could cause cell lethality. Mts knockdown cells were marked by green fluorescent protein expression. D and _D_′, a wing disc expressing _UAS-mts_RNAi was immunostained for Arm and Dll. Arrow in D indicates the destabilization of Arm and the arrow in _D_′ indicates the down-regulated Dll expression by mts RNAi. E and _E_′, a wing disc expressing UAS-mts by ptc-gal4 was immunostained with anti-Arm and Sen. Arrow in E indicates the elevation of Arm by the overexpression of Mts.
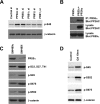
FIGURE 3.
Identification of PR55α as the regulatory B subunit that controls β-catenin phosphorylation. A, SW480 cells were transfected with siRNAs targeting several different regulatory subunits of PP2A. Negative control siRNA and PP2Ac siRNA were used as controls. Total β-catenin and phosphorylated β-catenin were analyzed by Western blot with anti-Ser(P)-45 and anti-β-catenin Abs. B, interaction between PR55α and PP2Ac. Myc-tagged PR55α was cotransfected with CS2 control plasmid or FLAG-tagged PP2Ac into HEK293T cells. PR55α protein was immunoprecipitated from cell lysates with an anti-Myc Ab. The presence of PP2Ac in the immunoprecipitated (IP) samples was analyzed by Western blot with an anti-FLAG Ab. The levels of PP2Ac and PR55α in the cell lysates were analyzed as control. C, SW480 cells were infected with lentiviruses that express control shRNA or _PR55_α shRNA. Stable cells were selected with puromycin. PR55α protein levels in these cells were analyzed by Western blot with an anti-PR55α Ab. Total β-catenin and phosphorylated β-catenin were analyzed with an anti-β-catenin Ab and phospho-specific Abs that recognize different phosphorylation sites of β-catenin. D, SW480 cells were treated with dimethyl sulfoxide or 10 n
m
OA. Total β-catenin and phosphorylated β-catenin were analyzed with an anti-β-catenin Ab and phospho-specific Abs against β-catenin.
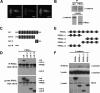
FIGURE 4.
PR55α interacts with β-catenin. A, localization of PR55α in SW480 cells. SW480 cells were stained with Abs against β-catenin (green) and PR55α (red). B, endogenous PR55α binds endogenous β-catenin. PR55α was immunoprecipitated (IP) with an anti-PR55α Ab and β-catenin was analyzed by Western blot with an anti-β-catenin Ab. The endogenous PR55α and β-catenin in the cell lysates were analyzed as control. C, schematic diagram of β-catenin deletion constructs. The black boxes are armadillo repeats. D, armadillo domains of β-catenin bind PR55α. HEK293T cells were cotransfected with Myc-tagged PR55α and Myc-tagged β-catenin or its mutants. PR55α was immunoprecipitated with an anti-PR55α Ab. β-Catenin proteins were analyzed by Western blot with an anti-Myc Ab. The expression of these proteins was analyzed by Western blot with an anti-Myc Ab. E, schematic diagram of PR55α deletion constructs. The hexagons are WD40 repeats. F, β-catenin interacts with multiple domains of PR55α. HEK293T cells were cotransfected with FLAG-tagged β-catenin and Myc-tagged PR55α or its mutants. PR55α proteins were immunoprecipitated with 9E10-conjugated beads and β-catenin was analyzed by Western blot with an anti-FLAG Ab. The levels of PR55α and β-catenin proteins in the cell lysates were analyzed as control.
FIGURE 5.
PR55α directly interacts with β-catenin and Axin and regulates PP2A-mediated β-catenin dephosphorylation. A, PR55α interacts with both Axin and β-catenin. HEK293T cells were cotransfected with Myc-tagged PR55α and FLAG-tagged Axin, or FLAG-tagged β-catenin. Axin and β-catenin were immunoprecipitated from the cell lysates using an anti-FLAG Ab. The presence of PR55α in the immunoprecipitated samples were analyzed with an anti-Myc Ab. The protein levels of Axin and β-catenin were analyzed with an anti-FLAG Ab. B, PR55α directly interacts with β-catenin and Axin. Purified histidine-tagged PP2Ac and PR55α were incubated with purified GST, GST-β-catenin, GST-Axin(Cat+S45K), and GST-Axin(G3+Cat), respectively. These GST-Axin fusion proteins have been described previously (4). GST fusion proteins were pulled down with glutathione-agarose beads. PP2Ac and PR55α were analyzed with anti-PP2Ac and anti-PR55α Abs. The binding between these GST proteins and CKIα and GSK3 were analyzed as controls. C, the C-terminal region of PR55α interacts with Axin. HEK293T cells were cotransfected with FLAG-tagged Axin and Myc-tagged PR55α or its mutants. PR55α proteins were immunoprecipitated with 9E10-conjugated beads and Axin was analyzed by Western blot with an anti-FLAG Ab. The levels of PR55α and Axin proteins in the cell lysates were analyzed as control. D, PR55α enhances Wnt signaling. Super8XTOPFlash was cotransfected with Wnt3A plus CS2 or Wnt3A plus PR55α constructs into HEK293T cells. Luciferase activity was analyzed and normalized. E, PR55α regulates β-catenin stability. Left panel, HEK293T cells were infected with lentiviruses that express control shRNA or _PR55_α shRNA. Stable cells were selected with puromycin. Cytoplasmic PR55α and β-catenin protein levels were analyzed by Western blot with anti-PR55α and anti-β-catenin Abs. Right panel, the HEK293T cell line contains _PR55_α shRNA (HEK293i) and the control HEK293T cell line were treated with control conditioned medium or Wnt3A-conditioned medium for 6 h. Cytoplasmic fractions were isolated from these cells. β-Catenin and PR55α levels were analyzed by Western blot using anti-β-catenin and anti-PR55α Ab. β-Tubulin was analyzed as a loading control. F, PP2A holoenzyme containing PR55α directly dephosphorylates β-catenin. GST-β-catenin was phosphorylated by CKI and GSK3 in vitro. Left panel, Myc-tagged PR55α and FLAG-tagged PP2Ac were cotransfected into HEK293T cells. HEK293T cells transfected with empty vector were used as control. The PR55α-PP2Ac complex was immunoprecipitated with 9E10-conjugated beads. The beads were resuspended in 1× phosphatase buffer and incubated with phosphorylated GST-β-catenin for 30 min. PR55α, PP2Ac, total β-catenin, and phosphorylated β-catenin were analyzed by Western blot. Right panel, PP2A holoenzyme containing FLAG-tagged PR55α was purified from HEK293T cells and incubated with phosphorylated β-catenin for 0, 10, 20, and 30 min. PR55α and the catalytic subunit of PP2A (PP2Ac) were analyzed by Western blot with anti-PR55α and anti-PP2Ac Abs. β-Catenin was analyzed by Western blot with Abs against phosphorylated β-catenin or total β-catenin.
Similar articles
- Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus.
Li X, Yost HJ, Virshup DM, Seeling JM. Li X, et al. EMBO J. 2001 Aug 1;20(15):4122-31. doi: 10.1093/emboj/20.15.4122. EMBO J. 2001. PMID: 11483515 Free PMC article. - Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex.
Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM. Luo W, et al. EMBO J. 2007 Mar 21;26(6):1511-21. doi: 10.1038/sj.emboj.7601607. Epub 2007 Feb 22. EMBO J. 2007. PMID: 17318175 Free PMC article. - Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex.
Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Gao ZH, et al. Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1182-7. doi: 10.1073/pnas.032468199. Epub 2002 Jan 29. Proc Natl Acad Sci U S A. 2002. PMID: 11818547 Free PMC article. - Use of okadaic acid to identify relevant phosphoepitopes in pathology: a focus on neurodegeneration.
Medina M, Avila J, Villanueva N. Medina M, et al. Mar Drugs. 2013 May 21;11(5):1656-68. doi: 10.3390/md11051656. Mar Drugs. 2013. PMID: 23697949 Free PMC article. Review. - The Protein Phosphatase PP2A Plays Multiple Roles in Plant Development by Regulation of Vesicle Traffic-Facts and Questions.
Máthé C, M-Hamvas M, Freytag C, Garda T. Máthé C, et al. Int J Mol Sci. 2021 Jan 19;22(2):975. doi: 10.3390/ijms22020975. Int J Mol Sci. 2021. PMID: 33478110 Free PMC article. Review.
Cited by
- PR55α-controlled protein phosphatase 2A inhibits p16 expression and blocks cellular senescence induction by γ-irradiation.
Palanivel C, Madduri LSV, Hein AL, Jenkins CB, Graff BT, Camero AL, Zhou S, Enke CA, Ouellette MM, Yan Y. Palanivel C, et al. Aging (Albany NY). 2024 Mar 4;16(5):4116-4137. doi: 10.18632/aging.205619. Epub 2024 Mar 4. Aging (Albany NY). 2024. PMID: 38441530 Free PMC article. - The scaffold protein AXIN1: gene ontology, signal network, and physiological function.
Qiu L, Sun Y, Ning H, Chen G, Zhao W, Gao Y. Qiu L, et al. Cell Commun Signal. 2024 Jan 30;22(1):77. doi: 10.1186/s12964-024-01482-4. Cell Commun Signal. 2024. PMID: 38291457 Free PMC article. Review. - LASS2 enhances chemosensitivity to cisplatin by inhibiting PP2A-mediated β-catenin dephosphorylation in a subset of stem-like bladder cancer cells.
Shi H, Tan Z, Duan B, Guo C, Li C, Luan T, Li N, Huang Y, Chen S, Gao J, Feng W, Xu H, Wang J, Fu S, Wang H. Shi H, et al. BMC Med. 2024 Jan 9;22(1):19. doi: 10.1186/s12916-023-03243-5. BMC Med. 2024. PMID: 38191448 Free PMC article. - A novel RIP1-mediated canonical WNT signaling pathway that promotes colorectal cancer metastasis via β -catenin stabilization-induced EMT.
Kang AR, Kim JL, Kim Y, Kang S, Oh SC, Park JK. Kang AR, et al. Cancer Gene Ther. 2023 Oct;30(10):1403-1413. doi: 10.1038/s41417-023-00647-6. Epub 2023 Jul 27. Cancer Gene Ther. 2023. PMID: 37500894 Free PMC article. - Phosphorylated PTTG1 switches its subcellular distribution and promotes β-catenin stabilization and subsequent transcription activity.
Zhang X, Wu N, Huang H, Li S, Liu S, Zhang R, Huang Y, Lyu H, Xiao S, Ali DW, Michalak M, Chen XZ, Zhou C, Tang J. Zhang X, et al. Oncogene. 2023 Aug;42(32):2439-2455. doi: 10.1038/s41388-023-02767-7. Epub 2023 Jul 3. Oncogene. 2023. PMID: 37400529
References
- Clevers H. (2006) Cell 127,469–480 - PubMed
- Logan C. Y., Nusse R. (2004) Annu. Rev. Cell. Dev. Biol. 20,781–810 - PubMed
- Moon R. T. (2005) Sci. STKE. 2005,cm1. - PubMed
- Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Cell 108,837–847 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK071976-01A2/DK/NIDDK NIH HHS/United States
- R01 DK071976/DK/NIDDK NIH HHS/United States
- R01GM079684/GM/NIGMS NIH HHS/United States
- R21 CA112007/CA/NCI NIH HHS/United States
- R21 CA112007-01/CA/NCI NIH HHS/United States
- R01DK071976/DK/NIDDK NIH HHS/United States
- R01 GM079684/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases