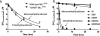On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex - PubMed (original) (raw)
On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex
Anamika Patel et al. J Biol Chem. 2009.
Abstract
Transcription in eukaryotic genomes depends on enzymes that regulate the degree of histone H3 lysine 4 (H3K4) methylation. The mixed lineage leukemia protein-1 (MLL1) is a member of the SET1 family of H3K4 methyltransferases and is frequently rearranged in acute leukemias. Despite sequence comparisons that predict that SET1 family enzymes should only monomethylate their substrates, mono-, di-, and trimethylation of H3K4 has been attributed to SET1 family complexes in vivo and in vitro. To better understand this paradox, we have biochemically reconstituted and characterized a five-component 200-kDa MLL1 core complex containing human MLL1, WDR5, RbBP5, Ash2L, and DPY-30. We demonstrate that the isolated MLL1 SET domain is a slow monomethyltransferase and that tyrosine 3942 of MLL1 prevents di- and trimethylation of H3K4. In contrast, a complex containing the MLL1 SET domain, WDR5, RbBP5, Ash2L, and DPY-30, displays a marked approximately 600-fold increase in enzymatic activity but only to the dimethyl form of H3K4. Single turnover kinetic experiments reveal that the reaction leading to H3K4 dimethylation involves the transient accumulation of a monomethylated species, suggesting that the MLL1 core complex uses a non-processive mechanism to catalyze multiple lysine methylation. We have also discovered that the non-SET domain components of the MLL1 core complex possess a previously unrecognized methyltransferase activity that catalyzes H3K4 dimethylation within the MLL1 core complex. Our results suggest that the mechanism of multiple lysine methylation by the MLL1 core complex involves the sequential addition of two methyl groups at two distinct active sites within the complex.
Figures
FIGURE 1.
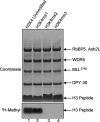
Purification and hydrodynamic characterization of MLL1 core complex components. a, Coomassie Brilliant Blue-stained SDS-PAGE showing the purified MLL1 core complex components. b, schematic representation showing the domain architecture of full-length MLL1 and the construct used in this investigation, which consisted of residues 3745–3969 (MLL3745). c, diffusion-free sedimentation coefficient distributions (c(s)) derived from sedimentation velocity data of individual MLL1 core complex components: MLL3745 (blue), WDR5 (pink), RbBP5 (green), Ash2L (purple), and DPY30 (red).
FIGURE 2.
Pairwise interactions within the MLL1 core complex. a, summary of pairwise interactions that could be observed by sedimentation velocity analytical ultracentrifugation. (+) interaction detected; (−) no interaction detected. b–e, c(s) distributions of sedimentation velocity data of binary complexes at the following concentrations: 7 μ
m
(solid black line), 3.5 μ
m
(dashed line), and 1.5 μ
m
(dotted line). b, MLL3745-WDR5 (reproduced from Ref. 45). c, WDR5-RbBP5; d, RbBP5-Ash2l; and e, Ash2L-DPY-30. f, schematic model summarizing the observed pairwise interactions and dissociation constants (Kd) within the MLL1 core complex.
FIGURE 3.
Characterization of the assembly and enzymatic activity of the MLL1 core complex. The left column from top to bottom shows the c(s) distributions from sedimentation velocity experiments after the addition of each component of the MLL1 core complex starting with: a, MLL3745 (M); d, MLL3745-WDR5 (M-W) (reproduced from Ref. 45). g, MLL3745-WDR5-RbBP5 (M-W-R); j, MLL3745-WDR5-RbBP5-Ash2L (M-W-R-A); and m, MLL3745-WDR5-RbBP5-Ash2L-DPY30 (M-W-R-A-D2). The center column from top to bottom (b, e, h, k, and n) shows MALDI-TOF mass spectrometry of enzymatic assays after 24 h. Each spectrum corresponds to the enzymatic activity of the complex in the c(s) panel on the left. The third column from top to bottom (c, f, i, l, and o) shows kinetic progression of methylation reactions catalyzed by the corresponding complexes on the left. Each time point represents the percentage of total integrated area for each species in MALDI-TOF assays.
FIGURE 4.
Determination of rate constants from single turnover progress curves measured by MALDI-TOF mass spectrometry. a, comparison of the overall rates of the reactions catalyzed by MLL3745 (M) in the presence and absence of MLL1-interacting proteins (W-R-A-D2). Solid lines were derived from fitting the decrease in the relative intensity of the unmodified histone H3 peptide peaks to Equation 1 under “Experimental Procedures.” b, single turnover progress curves for the reaction catalyzed by the MLL1 core complex (MWRA) from MALDI-TOF MS assays. The data for H3K4, H3K4me1, and H3K4me2 species were globally fitted to Equations 1–3 (see “Experimental Procedures”) using DynaFit. Error bars represent the ±S.E. from duplicate experiments. c, single turnover progress curves for the reaction catalyzed by the MLL1 core complex in the presence of DPY30 (MWRAD) globally fitted as in b. d, summary of rate constants (±S.E.) h−1 derived from globally fitting experimental data to Equations 1–3 under “Experimental Procedures.”
FIGURE 5.
The MLL1 core complex is a histone H3K4 dimethyltransferase. Lanes 1–4, comparison of the enzymatic activity of the MWRAD complex among histone H3 substrates that were unmodified (H3K4, lane 1) or previously monomethylated (H3K4me1, lane 2); dimethylated (H3K4me2, lane 3); or trimethylated (H3K4me3, lane 4) at H3K4. The upper panel shows a Coomassie Blue-stained SDS-PAGE gel of enzymatic reactions. The lower panel shows [3H]methyl incorporation into histone peptides as determined by fluorography.
FIGURE 6.
Tyrosine 3942 of MLL1 controls the product specificity of the MLL1 SET domain. a, the Phe/Tyr switch position of the MLL1 (Protein Data Bank (PDB) code: 2W5Z) and Dim5 (PDB code: 1PEG) SET domain active sites are superimposed. MLL1 is shown in magenta, and Dim5 is shown in green. The position of Tyr-3942 of MLL1 is indicated. The lysine substrate and AdoHcy cofactor are from the Dim5 ternary complex structure and are shown in yellow. b and c, MALDI-TOF mass spectrometry of histone H3 peptide methylation at various time points catalyzed by wild-type MLL3745 (MLL_3745(wt)) (b) and Y3942F MLL3745 (MLL_3745(Y3942F)) (c). d and e, kinetic progression of methylation reactions catalyzed by wild-type MLL3745 (d) and Y3942F MLL3745 (e). f, comparison of the enzymatic activities with different H3K4 substrates between the wild-type (left panel) and Y3942F (right panel) MLL3745 enzymes. The upper panels show Coomassie Brilliant Blue-stained gels of methylation reactions, and the lower panels show [3H]methyl incorporation into histone peptides as determined by fluorography.
FIGURE 7.
A non-SET domain component of the MLL1 core complex possesses a histone methyltransferase activity. a, enzymatic assays showing that the replacement of asparagine 3906 of MLL1 with alanine (MLL_3745(N3906A)_) abolishes the enzymatic activity of the isolated MLL1 SET domain (lanes 1 and 2). Lanes 3 and 4 show assays with a complex containing MLL3745(N3906A), WDR5, RbBP5, Ash2L, and DPY30. The upper panel shows a Coomassie Blue-stained gel of methylation reactions, and the lower panel shows the fluorogram of the same gel. b, c(s) distribution from sedimentation velocity analytical ultracentrifugation of the MLL1 core complex assembled with stoichiometric amounts of MLL3745(N3906A), WDR5, RbBP5, and Ash2L. c, MALDI-TOF mass spectrum of representative methylation reactions catalyzed by the MLL3745(N3906A) SET domain in the presence of WDR5, RbBP5, Ash2L, and DPY30 after 24 h.
FIGURE 8.
The isolated W-R-A-D2 subcomplex monomethylates lysine 4 of histone H3 and catalyzes H3K4 dimethylation within the MLL1 core complex. a, comparison of W-R-A-D2-catalyzed enzymatic activity among histone H3 peptides that were either unmodified (H3K4, lane 1) or monomethylated (H3K4me1, lane 2); dimethylated (H3K4me2, lane 3), or trimethylated (H3K4me3, lane 4) at H3K4. The upper panel shows Coomassie Blue-stained SDS-PAGE gel, and the lower panel shows [3H]methyl incorporation by fluorography. b, comparison of the enzymatic activity of the MLL1 core complex assembled with the N3906A MLL1 SET domain among histone H3 peptides as described in panel a above. (The protein band indicated by * is partially degraded MLL3745(N3906A).)
FIGURE 9.
The mechanism of multiple lysine methylation by the MLL1 core complex is distinct from that of the Y3942F MLL1 SET domain. a, comparison of reaction progress curves for the decrease in the relative intensity of unmodified histone H3 peptides catalyzed by the isolated wild-type and Y3942F MLL3745 SET domains as determined by MALDI-TOF mass spectrometry. The product specificity of each enzyme is indicated with an arrow. b, comparison of wild-type MLL3745-catalyzed reaction progress curves for unmodified H3K4 peptides in the presence and absence of MLL1-interacting proteins as determined by MALDI-TOF mass spectrometry.
FIGURE 10.
Proposed model for the mechanism of multiple lysine methylation by the MLL1 core complex. In this model, the MLL1 SET domain catalyzes monomethylation of histone H3 at site 1, which is followed by transfer of the monomethylated peptide to a second active site on the W-R-A-D2 subcomplex (site 2, white dashed oval), which then catalyzes dimethylation of histone H3. The white question marks in site 2 denote that fact that the catalytic motif of the W-R-A-D2 subcomplex is unknown. The rate constants are derived from the fitting of the data to this model (Fig. 4). Also shown is the interaction between WDR5 and Arg-3765 of the MLL1 Win motif, which was previously shown to be required for the assembly and dimethylation activity of the MLL1 core complex (45).
Similar articles
- Automethylation activities within the mixed lineage leukemia-1 (MLL1) core complex reveal evidence supporting a "two-active site" model for multiple histone H3 lysine 4 methylation.
Patel A, Vought VE, Swatkoski S, Viggiano S, Howard B, Dharmarajan V, Monteith KE, Kupakuwana G, Namitz KE, Shinsky SA, Cotter RJ, Cosgrove MS. Patel A, et al. J Biol Chem. 2014 Jan 10;289(2):868-84. doi: 10.1074/jbc.M113.501064. Epub 2013 Nov 14. J Biol Chem. 2014. PMID: 24235145 Free PMC article. - Structural basis for WDR5 interaction (Win) motif recognition in human SET1 family histone methyltransferases.
Dharmarajan V, Lee JH, Patel A, Skalnik DG, Cosgrove MS. Dharmarajan V, et al. J Biol Chem. 2012 Aug 10;287(33):27275-89. doi: 10.1074/jbc.M112.364125. Epub 2012 Jun 3. J Biol Chem. 2012. PMID: 22665483 Free PMC article. - Biochemical reconstitution and phylogenetic comparison of human SET1 family core complexes involved in histone methylation.
Shinsky SA, Monteith KE, Viggiano S, Cosgrove MS. Shinsky SA, et al. J Biol Chem. 2015 Mar 6;290(10):6361-75. doi: 10.1074/jbc.M114.627646. Epub 2015 Jan 5. J Biol Chem. 2015. PMID: 25561738 Free PMC article. - WRAD: enabler of the SET1-family of H3K4 methyltransferases.
Ernst P, Vakoc CR. Ernst P, et al. Brief Funct Genomics. 2012 May;11(3):217-26. doi: 10.1093/bfgp/els017. Epub 2012 May 30. Brief Funct Genomics. 2012. PMID: 22652693 Free PMC article. Review. - The Development of Inhibitors Targeting the Mixed Lineage Leukemia 1 (MLL1)-WD Repeat Domain 5 Protein (WDR5) Protein- Protein Interaction.
Ye X, Chen G, Jin J, Zhang B, Wang Y, Cai Z, Ye F. Ye X, et al. Curr Med Chem. 2020;27(33):5530-5542. doi: 10.2174/0929867326666190528080514. Curr Med Chem. 2020. PMID: 31132972 Review.
Cited by
- A high throughput scintillation proximity imaging assay for protein methyltransferases.
Ibanez G, Shum D, Blum G, Bhinder B, Radu C, Antczak C, Luo M, Djaballah H. Ibanez G, et al. Comb Chem High Throughput Screen. 2012 Jun 1;15(5):359-71. doi: 10.2174/138620712800194468. Comb Chem High Throughput Screen. 2012. PMID: 22256970 Free PMC article. - Epigenetics of hematopoiesis and hematological malignancies.
Hu D, Shilatifard A. Hu D, et al. Genes Dev. 2016 Sep 15;30(18):2021-2041. doi: 10.1101/gad.284109.116. Genes Dev. 2016. PMID: 27798847 Free PMC article. Review. - Hierarchical assembly of the MLL1 core complex regulates H3K4 methylation and is dependent on temperature and component concentration.
Namitz KEW, Tan S, Cosgrove MS. Namitz KEW, et al. J Biol Chem. 2023 Feb;299(2):102874. doi: 10.1016/j.jbc.2023.102874. Epub 2023 Jan 6. J Biol Chem. 2023. PMID: 36623730 Free PMC article. - Physical and functional interaction between SET1/COMPASS complex component CFP-1 and a Sin3S HDAC complex in C. elegans.
Beurton F, Stempor P, Caron M, Appert A, Dong Y, Chen RA, Cluet D, Couté Y, Herbette M, Huang N, Polveche H, Spichty M, Bedet C, Ahringer J, Palladino F. Beurton F, et al. Nucleic Acids Res. 2019 Dec 2;47(21):11164-11180. doi: 10.1093/nar/gkz880. Nucleic Acids Res. 2019. PMID: 31602465 Free PMC article. - The Histone H3 Lysine 4 Presenter WDR5 as an Oncogenic Protein and Novel Epigenetic Target in Cancer.
Lu K, Tao H, Si X, Chen Q. Lu K, et al. Front Oncol. 2018 Nov 14;8:502. doi: 10.3389/fonc.2018.00502. eCollection 2018. Front Oncol. 2018. PMID: 30488017 Free PMC article. Review.
References
- Litt M. D., Simpson M., Gaszner M., Allis C. D., Felsenfeld G. (2001) Science 293, 2453–2455 - PubMed
- Santos-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. E., Emre N. C., Schreiber S. L., Mellor J., Kouzarides T. (2002) Nature 419, 407–411 - PubMed
- Pokholok D. K., Harbison C. T., Levine S., Cole M., Hannett N. M., Lee T. I., Bell G. W., Walker K., Rolfe P. A., Herbolsheimer E., Zeitlinger J., Lewitter F., Gifford D. K., Young R. A. (2005) Cell 122, 517–527 - PubMed
- Wysocka J., Swigut T., Xiao H., Milne T. A., Kwon S. Y., Landry J., Kauer M., Tackett A. J., Chait B. T., Badenhorst P., Wu C., Allis C. D. (2006) Nature 442, 86–90 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials