Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration - PubMed (original) (raw)
Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration
Julie Anne Seguin et al. Neuropsychiatr Dis Treat. 2009.
Abstract
Disturbances of hippocampal plasticity, including impaired dendritic branching and reductions of neurogenesis, are provoked by stressful insults and may occur in depression. Although corticoids likely contribute to stressor-induced reductions of neurogenesis, other signaling messengers, including pro-inflammatory cytokines might also be involved. Accordingly, the present investigation assessed whether three proinflammatory cytokines, namely interleukin-1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha (TNF-alpha) (associated with depression) influenced cellular proliferation within the hippocampus. In this regard, systemic administration of TNF-alpha reduced 5-bromo-2-deoxyuridine (BrdU) labeling within the hippocampus, whereas IL-1beta and IL-6 had no such effect. However, repeated but not a single intra-hippocampal infusion of IL-6 and IL-1beta actually increased cellular proliferation and IL-6 infusion also enhanced microglial staining within the hippocampus. Yet, no changes in doublecortin expression were apparent, suggesting that the cytokine did not influence the birth of cells destined to become neurons. Essentially, the route of administration and chronicity of cytokine administration had a marked influence upon the nature of hippocampal alterations provoked, suggesting that cytokines may differentially regulate hippocampal plasticity in neuropsychiatric conditions.
Keywords: cytokine; depression; hippocampus; neuroplasticity; stressor.
Figures
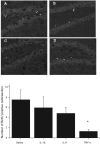
Figure 1
BrdU immunopositive cells are depicted in the photomicrographs of the rostral portion of hippocampal dentate gyrus of animals subjected to i.p. injection of A) saline, B) IL-1β (0.1 μg), C) TNF-α (1.0 μg) or (d) IL-6 (1.0 μg). There was a clear reduction of BrdU-labelled cells in mice that received TNF-α (panel C), relative to saline injected controls (panel A). The bottom bar graph displays the mean (± SEM) number of BrdU-positive cells per section and confirms the suppression of BrdU labeling following systemic administration of TNF-α. Notes: *p < 0.05 vs. saline-treated animals, 10× magnification.
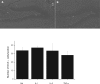
Figure 2
The photomicrographs (4× magnification) show doublecortin (DCX) positive immature neurons within the dentate gyrus of the rostral portion of dorsal hippocampus. There was no significant difference in DCX expression between mice that received i.p. saline A) or those treated with i.p. TNF-α B) The inset (upper right corner of panel A) reveals a higher magnification (20×) image of the DCX positive soma and projections from a saline-treated animal.
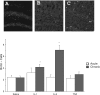
Figure 3
The photomicrographs depict BrdU-positive cells within the dentate gyrus following a single or repeated intra-hippocampal cytokine or saline infusion. Elevated BrdU staining within the dentate gyrus was observed in animals subjected to repeated intra-hippocampal infusion of either IL-1β (panel B) or IL-6 (panel C), relative to animals that received vehicle (panel A). The BrdU increase was particularly robust for the repeated IL-6 treatment. Quantification of mean (± SEM) number of BrdU-positive cells per section confirmed that repeated (grey bars) but not a single (white bars) intra-hippocampal infusion of IL-1β and IL-6 significantly increased BrdU staining, relative to animals that received infusion of saline. Notes: *p < 0.05 vs saline-treated animals, 10× magnification.
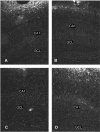
Figure 4
Photomicrographs of labeling for the microglial marker, CD11b, in animals subjected to a single or repeated intra-hippocampal infusions of saline or IL-6. The CD11b immunoreactivity was evident in the granule cell layer (GCL) of the dentate gyrus, as well as within the CA1 hippocampal region. Single (A) and repeated (B) infusion of saline provoked minimal CD11b staining in the GCL and CA1. Although the single IL-6 infusion (C) induced modest CD11b immunoreactivity, repeated infusion of the cytokine (D) produced the most marked increase of expression of the microglial marker within the GCL and CA1 areas of the hippocampus. 4× magnification.
Similar articles
- Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes.
Clarke M, Razmjou S, Prowse N, Dwyer Z, Litteljohn D, Pentz R, Anisman H, Hayley S. Clarke M, et al. Neuropharmacology. 2017 Jan;112(Pt A):210-220. doi: 10.1016/j.neuropharm.2016.04.021. Epub 2016 Apr 20. Neuropharmacology. 2017. PMID: 27106168 - Influence of continuous infusion of interleukin-1beta on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression.
Anisman H, Gibb J, Hayley S. Anisman H, et al. Psychopharmacology (Berl). 2008 Aug;199(2):231-44. doi: 10.1007/s00213-008-1166-z. Epub 2008 May 20. Psychopharmacology (Berl). 2008. PMID: 18491079 - Ifenprodil rapidly ameliorates depressive-like behaviors, activates mTOR signaling and modulates proinflammatory cytokines in the hippocampus of CUMS rats.
Yao Y, Ju P, Liu H, Wu X, Niu Z, Zhu Y, Zhang C, Fang Y. Yao Y, et al. Psychopharmacology (Berl). 2020 May;237(5):1421-1433. doi: 10.1007/s00213-020-05469-0. Epub 2020 Mar 4. Psychopharmacology (Berl). 2020. PMID: 32130432 - Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems.
Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Gądek-Michalska A, et al. Pharmacol Rep. 2013;65(6):1655-62. doi: 10.1016/s1734-1140(13)71527-5. Pharmacol Rep. 2013. PMID: 24553014 Review. - The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity.
Hayley S, Poulter MO, Merali Z, Anisman H. Hayley S, et al. Neuroscience. 2005;135(3):659-78. doi: 10.1016/j.neuroscience.2005.03.051. Epub 2005 Sep 8. Neuroscience. 2005. PMID: 16154288 Review.
Cited by
- Effects of human intravenous immunoglobulin on amyloid pathology and neuroinflammation in a mouse model of Alzheimer's disease.
Puli L, Pomeshchik Y, Olas K, Malm T, Koistinaho J, Tanila H. Puli L, et al. J Neuroinflammation. 2012 May 29;9:105. doi: 10.1186/1742-2094-9-105. J Neuroinflammation. 2012. PMID: 22642812 Free PMC article. - Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors.
Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Kesler S, et al. Brain Behav Immun. 2013 Mar;30 Suppl(0):S109-16. doi: 10.1016/j.bbi.2012.05.017. Epub 2012 Jun 12. Brain Behav Immun. 2013. PMID: 22698992 Free PMC article. - Perinatal Interactions between the Microbiome, Immunity, and Neurodevelopment.
Pronovost GN, Hsiao EY. Pronovost GN, et al. Immunity. 2019 Jan 15;50(1):18-36. doi: 10.1016/j.immuni.2018.11.016. Immunity. 2019. PMID: 30650376 Free PMC article. Review. - Loss of Cln5 causes altered neurogenesis in a mouse model of a childhood neurodegenerative disorder.
Savchenko E, Singh Y, Konttinen H, Lejavova K, Mediavilla Santos L, Grubman A, Kärkkäinen V, Keksa-Goldsteine V, Naumenko N, Tavi P, White AR, Malm T, Koistinaho J, Kanninen KM. Savchenko E, et al. Dis Model Mech. 2017 Sep 1;10(9):1089-1100. doi: 10.1242/dmm.029165. Epub 2017 Jul 21. Dis Model Mech. 2017. PMID: 28733362 Free PMC article. - Elocalcitol mitigates high-fat diet-induced microglial senescence via miR-146a modulation.
Chithanathan K, Jürgenson M, Ducena K, Remm A, Kask K, Rebane A, Tian L, Zharkovsky A. Chithanathan K, et al. Immun Ageing. 2024 Nov 22;21(1):82. doi: 10.1186/s12979-024-00485-6. Immun Ageing. 2024. PMID: 39578804 Free PMC article.
References
- Dunn AJ. The role of interleukin-1 and tumor necrosis factor alpha in the neurochemical and neuroendocrine responses to endotoxin. Brain Res Bull. 1992;29:807–812. - PubMed
- Hayley S, Anisman H. Multiple mechanisms of cytokine action in neurodegenerative and psychiatric states: Neurochemical and molecular substrates. Curr Pharm Des. 2005;11:947–962. - PubMed
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci. 1995;18:83–88. - PubMed
- Weigent DA, Carr DJ, Blalock JE. Bidirectional communication between the neuroendocrine and immune systems. common hormones and hormone receptors. Ann N Y Acad Sci. 1990;579:17–27. - PubMed
- Hayley S, Lacosta S, Merali Z, van Rooijen N, Anisman H. Central monoamine and plasma corticosterone changes induced by a bacterial endotoxin: Sensitization and cross-sensitization effects. Eur J Neurosci. 2001;13:1155–1165. - PubMed
LinkOut - more resources
Full Text Sources