The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores - PubMed (original) (raw)
The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores
Xiangang Zong et al. Pflugers Arch. 2009 Sep.
Abstract
Second messenger-induced Ca(2+)-release from intracellular stores plays a key role in a multitude of physiological processes. In addition to 1,4,5-inositol trisphosphate (IP(3)), Ca(2+), and cyclic ADP ribose (cADPR) that trigger Ca(2+)-release from the endoplasmatic reticulum (ER), nicotinic acid adenine dinucleotide phosphate (NAADP) has been identified as a cellular metabolite that mediates Ca(2+)-release from lysosomal stores. While NAADP-induced Ca(2+)-release has been found in many tissues and cell types, the molecular identity of the channel(s) conferring this release remained elusive so far. Here, we show that TPCN2, a novel member of the two-pore cation channel family, displays the basic properties of native NAADP-dependent Ca(2+)-release channels. TPCN2 transcripts are widely expressed in the body and encode a lysosomal protein forming homomers. TPCN2 mediates intracellular Ca(2+)-release after activation with low-nanomolar concentrations of NAADP while it is desensitized by micromolar concentrations of this second messenger and is insensitive to the NAADP analog nicotinamide adenine dinucleotide phosphate (NADP). Furthermore, TPCN2-mediated Ca(2+)-release is almost completely abolished when the capacity of lysosomes for storing Ca(2+) is pharmacologically blocked. By contrast, TPCN2-specific Ca(2+)-release is unaffected by emptying ER-based Ca(2+) stores. In conclusion, these findings indicate that TPCN2 is a major component of the long-sought lysosomal NAADP-dependent Ca(2+)-release channel.
Figures
Fig. 1
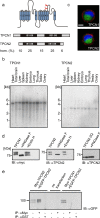
Properties of two-pore channels. aUpper panel Transmembrane topology of TPCN1 and TPCN2. The predicted N-glycosylation sites are marked by red forks. Lower panel Schematic representation of the primary sequence of TPCN1 and TPCN2. The degree of sequence identity within the N- and C-termini, the two transmembrane building blocks and the interdomain linker is indicated. b Mouse multiple tissue northern blots of TPCN1 (left panel) and TPCN2 (right panel) demonstrate expression in all tissues investigated. c EGFP-TPCN1 (upper panel) and EGFP-TPCN2 (lower panel) channels expressed in HEK293 cells are localized intracellularly (green TPCN channels; red membrane marker, blue nuclei; scale bar 5 µm). d Western blots with lysates from HEK293 cells containing myc-tagged TPCN1 (left panel), wild-type TPCN2 (middle) panel, and a glycosylation-deficient TPCN2 double-mutant (TPCN-2Q; right panel). Ten micrograms of protein were applied per lane. e Lysates of HEK293 cells cotransfected with myc-tagged TPCN2 and EGFP-tagged TPCN2 (lanes 1–3) or myc-tagged TPCN1 and EGFP-tagged TPCN2 (lanes 6–8) were immunoprecipitated with anti-myc antibody (lanes 2, 5, 7) or anti-GST (control, lanes 3 and 8), blotted and probed with anti-GFP. Lanes 1, 6 input, lane 4 negative control
Fig. 2
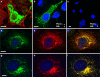
TPCN2 is localized in the lysosomes. Immunocytochemistry of TPCN2 in COS-7 cells. a Strong TPCN2 staining (green) is observed in intracellular compartments. By contrast, TPCN2 is absent from the plasma membrane (visualized in red by a specific maker). b After permeabilization, HA-tagged TPCN2 is detected intracellularily. c HA-tagged TPCN2 is not detected in the membrane of non permeabilized cells. d–f Colocalization of TPCN2 (green) and the ER marker protein calnexin (red). Most TPCN2-positive structures (d) costaine for endoplasmic reticulum (e), yielding yellow in the overlay (f). g–i Colocalization of TPCN2 (green) and lamp1 (red). Most TPCN2-positive structures (g) costained for lysosomes (h), yielding yellow in the overlay (i). (bars 10 µm)
Fig. 3
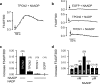
NAADP triggers Ca2+-release in HEK293 cells transfected with TPCN2. Application of 30 nM NAADP via the patch pipette induces Ca2+-release from internal stores in cells transfected with TPCN2-EGFP (a) but not in cells transfected with EGFP alone (b, upper panel) or cells transfected with TPCN1-EGFP (b, lower panel). In cells transfected with TPCN2-EGFP, NADP (30 nM; negative control) did not induce Ca2+-release (b, middle panel). The arrows in a, b indicate the start of cell perfusion. c Population data for experiments performed in (a, b). d Dose–response relationship of NAADP. All values are given as mean ± SEM. Number of cells measured is indicated in brackets in (c) and (d)
Fig. 4
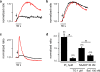
TPCN2 mediates NAADP-dependent Ca2+-release from lysosomes. a In HEK293 cells transfected with TPCN2-EGFP, preincubation (45 min) with 100 nM bafilomycin almost completely abolished NAADP (30 nM)-induced Ca2+-release (black line). Red line control without pretreatment. b In HEK293 cells transfected with TPCN2-EGFP, preincubation (15 min) with 1 µM thapsigargin did not reduce NAADP-sensitive Ca2+-release (black line). Red line same control as in (a). c Effect of thapsigargin (1 µM) on the IP3 (3 µM)-induced Ca2+-release. Experiments were performed either with (black line) or without (red line) thapsigargin preincubation (1 µM; 15 min). Arrows in (a–c) indicate the start of cell perfusion. d Population data for experiments shown in (a–c). Number of cells measured is indicated in brackets. Tg thapsigargin; Baf bafilomycin; IP3 Inositol-1,4,5-trisphosphate. Fluorescent ratio were normalized for better comparison
Similar articles
- Nicotinic acid adenine dinucleotide phosphate activates two-pore channel TPC1 to mediate lysosomal Ca2+ release in endothelial colony-forming cells.
Moccia F, Zuccolo E, Di Nezza F, Pellavio G, Faris PS, Negri S, De Luca A, Laforenza U, Ambrosone L, Rosti V, Guerra G. Moccia F, et al. J Cell Physiol. 2021 Jan;236(1):688-705. doi: 10.1002/jcp.29896. Epub 2020 Jun 24. J Cell Physiol. 2021. PMID: 32583526 - NAADP mobilizes calcium from acidic organelles through two-pore channels.
Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. Calcraft PJ, et al. Nature. 2009 May 28;459(7246):596-600. doi: 10.1038/nature08030. Epub 2009 Apr 22. Nature. 2009. PMID: 19387438 Free PMC article. - NAADP mobilizes calcium from the endoplasmic reticular Ca(2+) store in T-lymphocytes.
Steen M, Kirchberger T, Guse AH. Steen M, et al. J Biol Chem. 2007 Jun 29;282(26):18864-71. doi: 10.1074/jbc.M610925200. Epub 2007 Apr 19. J Biol Chem. 2007. PMID: 17446167 - Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling.
Lee HC. Lee HC. Sci China Life Sci. 2011 Aug;54(8):699-711. doi: 10.1007/s11427-011-4197-3. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786193 Review. - Physiological roles of NAADP-mediated Ca2+ signaling.
Galione A, Parrington J, Funnell T. Galione A, et al. Sci China Life Sci. 2011 Aug;54(8):725-32. doi: 10.1007/s11427-011-4207-5. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786195 Review.
Cited by
- Better Together: Interorganellar Communication in the Regulation of Proteostasis.
Kohler A, Kohler V. Kohler A, et al. Contact (Thousand Oaks). 2024 Oct 8;7:25152564241272245. doi: 10.1177/25152564241272245. eCollection 2024 Jan-Dec. Contact (Thousand Oaks). 2024. PMID: 39385949 Free PMC article. Review. - cAMP-dependent recruitment of acidic organelles for Ca2+ signaling in the salivary gland.
Imbery JF, Bhattacharya S, Khuder S, Weiss A, Goswamee P, Iqbal AK, Giovannucci DR. Imbery JF, et al. Am J Physiol Cell Physiol. 2016 Nov 1;311(5):C697-C709. doi: 10.1152/ajpcell.00010.2016. Epub 2016 Sep 7. Am J Physiol Cell Physiol. 2016. PMID: 27605449 Free PMC article. - TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes.
Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. Wang X, et al. Cell. 2012 Oct 12;151(2):372-83. doi: 10.1016/j.cell.2012.08.036. Cell. 2012. PMID: 23063126 Free PMC article. - The role of transient receptor potential cation channels in Ca2+ signaling.
Gees M, Colsoul B, Nilius B. Gees M, et al. Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a003962. doi: 10.1101/cshperspect.a003962. Epub 2010 Sep 22. Cold Spring Harb Perspect Biol. 2010. PMID: 20861159 Free PMC article. - TPC Functions in the Immune System.
Steiner P, Arlt E, Boekhoff I, Gudermann T, Zierler S. Steiner P, et al. Handb Exp Pharmacol. 2023;278:71-92. doi: 10.1007/164_2022_634. Handb Exp Pharmacol. 2023. PMID: 36639434
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1096/fj.05-5538fje', 'is_inner': False, 'url': 'https://doi.org/10.1096/fj.05-5538fje'}, {'type': 'PubMed', 'value': '16585058', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16585058/'}\]}
- Beck A, Kolisek M, Bagley LA, Fleig A, Penner R (2006) Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. Faseb J 20:962–964 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.M203224200', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.m203224200'}, {'type': 'PubMed', 'value': '12223470', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12223470/'}\]}
- Berridge G, Dickinson G, Parrington J, Galione A, Patel S (2002) Solubilization of receptors for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate. J Biol Chem 277:43717–43723 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/35036035', 'is_inner': False, 'url': 'https://doi.org/10.1038/35036035'}, {'type': 'PubMed', 'value': '11413485', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11413485/'}\]}
- Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1161/01.RES.0000047507.22487.85', 'is_inner': False, 'url': 'https://doi.org/10.1161/01.res.0000047507.22487.85'}, {'type': 'PubMed', 'value': '12480818', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12480818/'}\]}
- Boittin FX, Galione A, Evans AM (2002) Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ Res 91:1168–1175 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1073/pnas.85.21.7972', 'is_inner': False, 'url': 'https://doi.org/10.1073/pnas.85.21.7972'}, {'type': 'PMC', 'value': 'PMC282335', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC282335/'}, {'type': 'PubMed', 'value': '2973058', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2973058/'}\]}
- Bowman EJ, Siebers A, Altendorf K (1988) Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A 85:7972–7976 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous