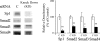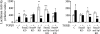Transforming growth factor-beta1 up-regulation of human alpha(1)(I) collagen is mediated by Sp1 and Smad2 transacting factors - PubMed (original) (raw)
Transforming growth factor-beta1 up-regulation of human alpha(1)(I) collagen is mediated by Sp1 and Smad2 transacting factors
Polina Sysa et al. DNA Cell Biol. 2009 Sep.
Abstract
Hepatic fibrosis results from excessive deposition of type I collagen. The roles of Smads in mediating the effect of transforming growth factor-beta1 (TGFbeta1) on activation of the alpha(1)(I) collagen promoter were determined. Smads bind in association with Sp1 to the CC(GG)-rich TGFbeta1 responsive element of the promoter that lacks the classical Smad recognition element, and enhance binding of Sp1. In transfection experiments, TGFbeta1 activated a proximal promoter, but not promoters mutated at sites that prevented Sp1 binding. Sp1 alone or the combination of Smad2 and Smad4 activated the promoter in transfected human LX-2 stellate cells. Sp1 or Smad2 knockdowns with siRNAs prevented the effect of TGFbeta1 in enhancing the promoter. In conclusion, this study shows that Smads bind in association with Sp1 to the CC(GG)-rich TGFbeta1 responsive element of the human alpha(1)(I) collagen promoter that lacks the classical Smad recognition element, thus enhancing the binding of Sp1 and in this manner activating the collagen promoter.
Figures
FIG. 1.
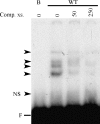
Mutational analysis of the TGFβ1 responsive element of the α1(I) collagen promoter. EMSA was performed with 1 pg of the labeled Sp1.1 oligonucleotide and 8 μg of nuclear extracts form LX-2 human stellate cells. The arrowheads indicate the protein–DNA complexes formed. The boldface letters indicate the mutated sequences in each mutant. NS, nonspecific band; F, the free probe; TGFβ1, transforming growth factor β1; EMSA, electrophoretic mobility shift assay.
- WT: 5′-CTTCCCTCCTCCTCCCCCTCTCC-3′
- M1: 5′-CTTTTCTCCTCCTCCCCCTCTCC-3′
- M2: 5′-CTTCCTACCTCCTCCCCCTCTCC-3′
- M3: 5′-CTTCCCTTTTCCTCCCCCTCTCC-3′
- M4: 5′-CTTCCCTCCATCTCCCCCTCTCC-3′
- M5: 5′-CTTCCCTCCTTTACCCCCTCTCC-3′
- M6: 5′-CTTCCCTCCTCCTTTCCCTCTCC-3′
- M7: 5′-CTTCCCTCCTCCTCCTTCTCTCC-3′
- M8: 5′-CTTCCCTCCTCCTCCCCTACTCC-3′
- M9: 5′-CTTCCCTCCTCCTCCCCCTTACC-3′
FIG. 2.
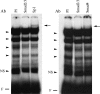
EMSA showing binding of nuclear extracts form LX-2 stellate cells to the wild-type (wt) Sp1.1 oligonucleotide in the presence of excess competing unlabeled oligonucleotide. The complexes formed are indicated by the arrowheads. NS indicates nonspecific band; F indicates the position of the free probe.
FIG. 3.
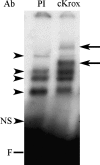
EMSA with supershift showing binding of nuclear extracts form LX-2 stellate cells to the Sp1.1 oligonucleotide. The EMSAs were performed with 10 μg of nuclear extract and the labeled oligonucleotide. For supershifts the antibodies (Ab) to Smad2/3, Smad4, and Sp1 (1:200 dilution) or preimmune serum (PI) were added to the reaction mixture. The arrowheads indicate the protein–DNA complexes formed. The arrows indicate the location of the supershifted complexes. NS indicates a nonspecific band; F indicates the position of the free probe.
FIG. 4.
EMSA with supershift showing binding of nuclear extracts form LX-2 stellate cells to the Sp1.1 oligonucleotide. The EMSAs were performed with 10 μg of nuclear extract and the labeled oligonucleotide. For supershifts the antibody (Ab) to cKrox (1:200 dilution) or preimmune serum (PI) was added to the reaction mixture. The arrowheads indicate the protein–DNA complexes formed. The arrows indicate the location of the supershifted complexes. NS indicates a nonspecific band; F indicates the position of the free probe.
FIG. 5.
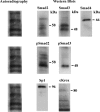
Autoradiography and Western blot showing the presence of Smad2, Smad3, Smad4, pSmad2, pSmad3, Sp1, and cKrox in nuclear extract from TGFβ1-treated LX-2 cells UV crosslinked to the labeled Sp1.1 oligonucleotide. The cells were exposed to TGFβ1 (10 ng/mL) for 24 h. The blots were exposed to film to determine the UV binding of the proteins and incubated with antibodies to the Smads, Sp1, and cKrox. The location of the bound proteins and their molecular mass (kDa) obtained from protein standards is shown.
FIG. 6.
Chromatin immunoprecipitation (ChIP) assay of Sp1 and Smad binding. One- (1×) and two-step (2×) cross-linked DNA–protein complexes were immunoprecipitated with antibodies to Sp1, Smad 2/3, and Smad4. Negative control was performed with purified rabbit IgG. The covalent linkage was reversed, and the precipitated double-stranded DNA was amplified to the region −199 to −93 (106 bp) of the α1(I) collagen promoter.
FIG. 7.
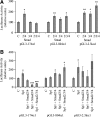
Effect of Smad expression vectors (pRK5F-Smad2, pRK5F-Smad3, and pRK-DPC4–Flag [Smad4]) on the activity of α1(I) collagen promoters in transfected LX-2 cells in (A) the absence and (B) the presence of the Sp1 expression vector (pPacSp1). Luciferase activity is expressed as the percentages of the control empty vectors, pRK5 (for Smads), pPadh (for Sp1), or their combination. The data are expressed as means ± SE of six determinations. *p < 0.05 versus control; **p < 0.01 versus control; ***p < 0.001 versus control.
FIG. 8.
Effect of Smad expression vectors (pRK5F-Smad2, pRK5F-Smad3, and pRK-DPC4–Flag [Smad4]) on the activity of α1(I) collagen promoters in transfected Drosophila Schneider cells in the absence and the presence of the Sp1 expression vector (pPacSp1). Luciferase activity is expressed as the percentages of the control empty vectors, pRK5 (for Smads), pPadh (for Sp1), or their combination. The data are expressed as means ± SE of six determinations. *p < 0.05 versus control; **p < 0.01 versus control; ***p < 0.001 versus control.
FIG. 9.
Effects of Sp1 and Smad siRNAs on protein expression of Sp1 and Smads determined by Western blot in the presence of TGFβ1 (10 ng/mL). C, negative control siRNA. KD, knock down. The values are expressed as means ± SE of relative densitometry of six measurements per group *p < 0.05 versus control; **p < 0.01 versus control.
FIG. 10.
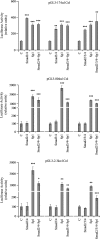
Effects of selective inhibition of Sp1, Smad2, Smad3, and Smad4 by siRNAs on the activity of the cotransfected pGL3-2.3k α1 wt collagen promoter in LX-2 cells treated or not treated with TGFβ1 (10 ng/mL). C, negative control siRNA. KD, knock down. The data are expressed as means ± SE of six determinations. *p < 0.05 versus respective group not treated with TGFβ1; **p < 0.01 versus respective group not treated with TGFβ1; X_p_ < 0.05 versus control not treated with TGFβ1; XX_p_ < 0.01 versus control not treated with TGFβ1; XXX_p_ < 0.01versus control not treated with TGFβ1.
FIG. 11.
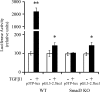
Effect of TGFβ1 on the α1(I) collagen promoter in _Smad3_−/− (Smad3 KO) mouse embryonic fibroblasts (MEF). The pGL3-2.3k α1 wt promoter and the p3TP-lux reporter, a TGFβ1 inducible reporter containing three TGFβ1-binding elements, were transfected into wt and Smad3−/− MEF. The cells were exposed or not exposed to TGFβ1 (10 ng/mL) for 24 h. Luciferase activity is expressed as a percentage of control. Data are presented as means ± SE of six determinations. *p < 0.05 versus control without TGFβ1; **p < 0.001 versus control without TGFβ1.
Similar articles
- Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription.
Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Zhang W, et al. J Biol Chem. 2000 Dec 15;275(50):39237-45. doi: 10.1074/jbc.M003339200. J Biol Chem. 2000. PMID: 11007770 - Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells.
Poncelet AC, Schnaper HW. Poncelet AC, et al. J Biol Chem. 2001 Mar 9;276(10):6983-92. doi: 10.1074/jbc.M006442200. Epub 2000 Dec 12. J Biol Chem. 2001. PMID: 11114293 - Tenascin-C upregulation by transforming growth factor-beta in human dermal fibroblasts involves Smad3, Sp1, and Ets1.
Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Jinnin M, et al. Oncogene. 2004 Mar 4;23(9):1656-67. doi: 10.1038/sj.onc.1207064. Oncogene. 2004. PMID: 15001984 - Activation of TGF-β1-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis.
Li HY, Ju D, Zhang DW, Li H, Kong LM, Guo Y, Li C, Wang XL, Chen ZN, Bian H. Li HY, et al. Sci Rep. 2015 Nov 12;5:16552. doi: 10.1038/srep16552. Sci Rep. 2015. PMID: 26559755 Free PMC article. - News from DNA and cell biology.
Handelsman J. Handelsman J. DNA Cell Biol. 2009 Sep;28(9):423. doi: 10.1089/dna.2009.1508. DNA Cell Biol. 2009. PMID: 19708814 No abstract available.
Cited by
- Characterisation of feline renal cortical fibroblast cultures and their transcriptional response to transforming growth factor β1.
Lawson JS, Syme HM, Wheeler-Jones CPD, Elliott J. Lawson JS, et al. BMC Vet Res. 2018 Mar 9;14(1):76. doi: 10.1186/s12917-018-1387-2. BMC Vet Res. 2018. PMID: 29523136 Free PMC article. - Antifibrotic effects of roscovitine in normal and scleroderma fibroblasts.
Steinman RA, Robinson AR, Feghali-Bostwick CA. Steinman RA, et al. PLoS One. 2012;7(11):e48560. doi: 10.1371/journal.pone.0048560. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185265 Free PMC article. - The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury.
Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. Kriegel AJ, et al. Physiol Genomics. 2012 Feb 27;44(4):237-44. doi: 10.1152/physiolgenomics.00141.2011. Epub 2012 Jan 3. Physiol Genomics. 2012. PMID: 22214600 Free PMC article. Review. - Expression of human frataxin is regulated by transcription factors SRF and TFAP2.
Li K, Singh A, Crooks DR, Dai X, Cong Z, Pan L, Ha D, Rouault TA. Li K, et al. PLoS One. 2010 Aug 20;5(8):e12286. doi: 10.1371/journal.pone.0012286. PLoS One. 2010. PMID: 20808827 Free PMC article. - Beneficial effects of adiponectin on periodontal ligament cells under normal and regenerative conditions.
Nokhbehsaim M, Keser S, Nogueira AV, Cirelli JA, Jepsen S, Jäger A, Eick S, Deschner J. Nokhbehsaim M, et al. J Diabetes Res. 2014;2014:796565. doi: 10.1155/2014/796565. Epub 2014 Jul 13. J Diabetes Res. 2014. PMID: 25121107 Free PMC article.
References
- Aparicio O. Geisberg J.V. Sekinger E. Yang A. Moqtaderi Z. Struhl K. Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo. Curr Protoc Mol Biol. 2005;Chapter 21:21.3.1–21.3.33. - PubMed
- Attisano L. Wrana J.L. Signal transduction by the TGF-β superfamilty. Science. 2002;296:1646–1647. - PubMed
- Chen S.J. Artlett C.M. Jimenez A. Varga J. Modulation of human α1(I) procollagen gene activity by interaction with Sp1 and Sp3 transcription factors in vitro. Gene. 1998;215:101–110. - PubMed
- Chen S.J. Yuan W. Lo S. Trojanowska M. Varga J. Interaction of Smad3 with a proximal Smad-binding element of the human α2 (I) procollagen gene promoter required for transcriptional activation of TGF-β. J Cell Physiol. 2000;183:381–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AA000626/AA/NIAAA NIH HHS/United States
- 2464388/PHS HHS/United States
- R01 AA000626/AA/NIAAA NIH HHS/United States
- 2 T32 AA07467/AA/NIAAA NIH HHS/United States
- T32 AA007467/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous