A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen - PubMed (original) (raw)
A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen
Chirajyoti Deb et al. PLoS One. 2009.
Abstract
Background: Mycobacterium tuberculosis (Mtb) becomes dormant and phenotypically drug resistant when it encounters multiple stresses within the host. Inability of currently available drugs to kill latent Mtb is a major impediment to curing and possibly eradicating tuberculosis (TB). Most in vitro dormancy models, using single stress factors, fail to generate a truly dormant Mtb population. An in vitro model that generates truly dormant Mtb cells is needed to elucidate the metabolic requirements that allow Mtb to successfully go through dormancy, identify new drug targets, and to screen drug candidates to discover novel drugs that can kill dormant pathogen.
Methodology/principal findings: We developed a novel in vitro multiple-stress dormancy model for Mtb by applying combined stresses of low oxygen (5%), high CO(2) (10%), low nutrient (10% Dubos medium) and acidic pH (5.0), conditions Mtb is thought to encounter in the host. Under this condition, Mtb stopped replicating, lost acid-fastness, accumulated triacylglycerol (TG) and wax ester (WE), and concomitantly acquired phenotypic antibiotic-resistance. Putative neutral lipid biosynthetic genes were up-regulated. These genes may serve as potential targets for new antilatency drugs. The triacylglycerol synthase1 (tgs1) deletion mutant, with impaired ability to accumulate TG, exhibited a lesser degree of antibiotic tolerance and complementation restored antibiotic tolerance. Transcriptome analysis with microarray revealed the achievement of dormant state showing repression of energy generation, transcription and translation machineries and induction of stress-responsive genes. We adapted this model for drug screening using the Alamar Blue dye to quantify the antibiotic tolerant dormant cells.
Conclusions/significance: The new in vitro multiple stress dormancy model efficiently generates Mtb cells meeting all criteria of dormancy, and this method is adaptable to high-throughput screening for drugs that can kill dormant Mtb. A critical link between storage-lipid accumulation and development of phenotypic drug-resistance in Mtb was established. Storage lipid biosynthetic genes may be appropriate targets for novel drugs that can kill latent Mtb.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
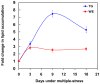
Figure 1. Accumulation of storage lipids by Mtb under multiple-stress in vitro.
Mtb was subjected to a combination of four stresses - high CO2, low O2, acidic pH and nutrient starvation. Total lipids were extracted at 0, 3, 9 and 18 days and resolved on silica-TLC using hexane-diethyl ether-formic acid (90∶10∶1, v/v/v). Lipids were visualized by charring after spraying with dichromate-sulfuric acid and quantified by densitometry using Alpha Innotech Gel documentation system and AlphaImager 2200 software (Alpha Innotech, USA). TG, triglycerides; WE, wax esters.
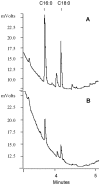
Figure 2. WE and TG accumulated by Mtb under multiple-stress is composed mainly of C16:0 and C18:0 fatty acids.
WE (A) and TG (B) accumulated by Mtb after 18 days under MS were trans-esterified and analyzed by capillary gas chromatography on Varian CP-TAP CB column using a temperature program as described in Materials and Methods.
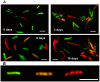
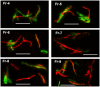
Figure 3. Accumulation of lipid bodies and loss of acid-fastness in Mtb cells under multiple-stress.
(A), Acid-fast staining cells (green) decreased and lipid body staining cells (red) increased with time under multiple-stress. Cells were stained with Auramine-O (acid-fast stain) and Nile Red (neutral lipid stain) and examined by confocal laser scanning microscopy (Leica TCS SP5) at the same laser intensity for all the samples with Z-stacking to get the depth of the scan field. Scanned samples were analyzed by LAS AF software for image projection. Overlaid images of the dual-stained Mtb are shown. Bar = 4 µM. (B), Magnified view of three different Mtb cells, representing three different subsets of Mtb cells in terms of acid-fast and neutral lipid staining property, observed in the Mtb population under multiple-stress: only acid-fast positive without any Nile Red stain (green), both acid-fast and lipid stain positive (orange yellow) and acid-fast negative cells with only Nile Red staining lipid bodies (right). The only acid-fast stain (green) positive cells gradually decreased and the other two types steadily increased during multiple-stress treatment. These cells selected from a day 9 sample were stained with both dyes and examined by confocal scanning as stated above in (A). Bar = 5 µM.
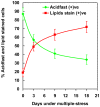
Figure 4. Increase in the percentage of lipid-stained cells and decrease in the percentage of acid-fast stained cells in Mtb culture subjected to in vitro multiple-stress.
Number of Auramine-O stained acid-fast positive (green) and Nile Red stained lipid body positive cells (red) were counted from multiple microscopic scans as presented in the figure 3.
Figure 5. Decrease in buoyant density of Mtb cells subjected to multiple-stress.
Mtb cells subjected to the multiple-stresses were placed on the preformed gradient and centrifuged at 400 g for 20 min. The center tube is a 3 day cell sample mixed with density marker (M) beads. Percoll gradients were self-formed by centrifugation from a starting solution with a density (ρ) of 1.0925 gm/ml. The densities of selected bead layers (ρ, in gm/ml) are given on the right, and the positions of one ml fractions collected for analyses are at the left. Numbers below the tubes indicate the number of days under multiple-stress.
Figure 6. Auramine-O (green) and Nile Red staining of Mtb cells in Percoll gradient fractions of Mtb culture after 18 day in multiple-stress.
Density gradient fractionation was performed as described in figure 5. Changes in acid-fast property, lipid accumulation and elongated cells with cording were observed. Nile Red staining Mtb cells concentrated in higher fraction numbers at lighter density. No cells were detected in fractions 1, 2 and only a few were detected in fraction 3. Fr, fraction; fraction numbers ascending from the bottom of the tube to the top. Bar = 5 µM.
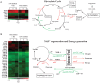
Figure 7. Microarray analysis demonstrated changes in expression of genes involved in glyoxylate cycle and energy metabolism.
(A), The expression ratio of genes involved in glyoxylate shunt cycle was shown in the red-green-display according to the log2-tranformed color code. Experimental time-points were shown at the top of the column. Genes were selected based on their annotation in TubercuList database, and grouped into those that were either regulated at least one of the time-points under multiple- stress condition. (B), Energy generation and NAD regeneration under multiple-stress. Genes involved in energy generation were selected based on their annotation. Red denotes induction and green denotes repression.
Figure 8. Functional clustering of Mtb genes under multiple-stress revealed nutrient starvation made major contribution to the number of genes that show changes in expression.
(A), Microarray data of Mtb gene expression under multiple-stress compared to the respective expression data for selected genes reported for nutrient-starvation- , hypoxic- , , and low pH-response . Expression ratios were averaged, log2-transformed, and displayed according to the color code at the bottom of the each column. Experimental time-points were indicated at the top of the each column. The Euclidean average linkage clustering (standard z-transformed) was performed to generate gene trees shown at the left side of each column. MS, Multiple-stress. (B), Venn diagrams showing the number of overlapping and unique set of genes modulated more than 1.8-fold at any time-points under multiple-stress condition. Induced or repressed genes were selected to categorize based on stress-response in red circle or green circle to indicate gene induction or repression, respectively.
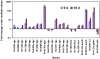
Figure 9. Real-time Taqman RT-PCR measurement of transcripts levels of selected genes potentially involved in dormancy and synthesis of storage lipids (TG and WE) in Mtb H37Rv under multiple-stress.
Relative quantitation method (ddCt) was used with the 7500 Fast real time system and analysis was done using SDS v1.4 software of Applied Biosystems Inc. sigA was used as the endogenous control to normalize expression values and samples of starter cultures (day 0) were used as calibrator to calculate the fold induction. Y axis is in log scale.
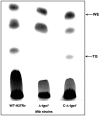
Figure 10. Loss of TG accumulation in Δ-tgs1 (Δ-Rv3130c) under multiple-stress (18 days) and its restoration by complementation.
Equal amount of lipid for each strain was loaded to Silica-TLC and resolved using hexane-diethyl ether-formic acid (90∶10∶1, v/v/v) solvent system. Lipids were visualized by charring for 10 min at 180°C after spraying with dichromate-sulfuric acid. WE, wax ester; TG, triglyceride; WT-H37Rv, Mtb H37Rv; Δ-_tgs_1, tgs1 deletion mutant of Mtb H37Rv; C-Δ-_tgs_1, tgs1 complemented strain of Δ-_tgs_1 mutant.
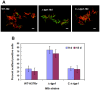
Figure 11. (A), Diminished ‘loss of acid-fastness’ in the Δ-tgs1 mutant population under multiple-stress.
WT-H37Rv, Δ-tgs1 mutant and C-Δ-tgs1 cells were stained with Auramine-O (acid-fast) and Nile Red (neutral lipid) after 18 days under multiple-stress. Bar = 4 µM. (B), Percent acid-fast stain positive cells observed in different Mtb strains under multiple-stress for 9 and 18 days. WT-H37Rv, wild type Mtb H37RV; Δ-tgs1, tgs1 deletion mutant of WT-H37Rv; C-Δ-tgs1, tgs1 complemented strain of Δ-tgs1.
Similar articles
- Wax ester synthesis is required for Mycobacterium tuberculosis to enter in vitro dormancy.
Sirakova TD, Deb C, Daniel J, Singh HD, Maamar H, Dubey VS, Kolattukudy PE. Sirakova TD, et al. PLoS One. 2012;7(12):e51641. doi: 10.1371/journal.pone.0051641. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23272127 Free PMC article. - Human granuloma in vitro model, for TB dormancy and resuscitation.
Kapoor N, Pawar S, Sirakova TD, Deb C, Warren WL, Kolattukudy PE. Kapoor N, et al. PLoS One. 2013;8(1):e53657. doi: 10.1371/journal.pone.0053657. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308269 Free PMC article. - A mathematical representation of the development of Mycobacterium tuberculosis active, latent and dormant stages.
Magombedze G, Mulder N. Magombedze G, et al. J Theor Biol. 2012 Jan 7;292:44-59. doi: 10.1016/j.jtbi.2011.09.025. Epub 2011 Sep 29. J Theor Biol. 2012. PMID: 21968442 - Modeling of Mycobacterium tuberculosis dormancy in bacterial cultures.
Batyrshina YR, Schwartz YS. Batyrshina YR, et al. Tuberculosis (Edinb). 2019 Jul;117:7-17. doi: 10.1016/j.tube.2019.05.005. Epub 2019 May 23. Tuberculosis (Edinb). 2019. PMID: 31378272 Review. - Drug targets in dormant Mycobacterium tuberculosis: can the conquest against tuberculosis become a reality?
Gupta VK, Kumar MM, Singh D, Bisht D, Sharma S. Gupta VK, et al. Infect Dis (Lond). 2018 Feb;50(2):81-94. doi: 10.1080/23744235.2017.1377346. Epub 2017 Sep 21. Infect Dis (Lond). 2018. PMID: 28933243 Review.
Cited by
- The Benefits of Toxicity: M. smegmatis VapBC TA Module Is Induced by Tetracycline Exposure and Promotes Survival.
Zamakhaev M, Bespyatykh J, Goncharenko A, Shumkov M. Zamakhaev M, et al. Microorganisms. 2023 Nov 26;11(12):2863. doi: 10.3390/microorganisms11122863. Microorganisms. 2023. PMID: 38138007 Free PMC article. - Human mesenchymal stem cell based intracellular dormancy model of Mycobacterium tuberculosis.
Singh VK, Mishra A, Bark S, Mani A, Subbian S, Hunter RL, Jagannath C, Khan A. Singh VK, et al. Microbes Infect. 2020 Oct;22(9):423-431. doi: 10.1016/j.micinf.2020.05.015. Epub 2020 Jun 17. Microbes Infect. 2020. PMID: 32562667 Free PMC article. - Screening a library of 1600 adamantyl ureas for anti-Mycobacterium tuberculosis activity in vitro and for better physical chemical properties for bioavailability.
Scherman MS, North EJ, Jones V, Hess TN, Grzegorzewicz AE, Kasagami T, Kim IH, Merzlikin O, Lenaerts AJ, Lee RE, Jackson M, Morisseau C, McNeil MR. Scherman MS, et al. Bioorg Med Chem. 2012 May 15;20(10):3255-62. doi: 10.1016/j.bmc.2012.03.058. Epub 2012 Mar 31. Bioorg Med Chem. 2012. PMID: 22522007 Free PMC article. - Understanding the contribution of metabolism to Mycobacterium tuberculosis drug tolerance.
Samuels AN, Wang ER, Harrison GA, Valenta JC, Stallings CL. Samuels AN, et al. Front Cell Infect Microbiol. 2022 Aug 22;12:958555. doi: 10.3389/fcimb.2022.958555. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36072222 Free PMC article. Review. - Wax ester synthesis is required for Mycobacterium tuberculosis to enter in vitro dormancy.
Sirakova TD, Deb C, Daniel J, Singh HD, Maamar H, Dubey VS, Kolattukudy PE. Sirakova TD, et al. PLoS One. 2012;7(12):e51641. doi: 10.1371/journal.pone.0051641. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23272127 Free PMC article.
References
- World Health Organization. Tuberculosis facts. 2008:5–26.
- Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. - PubMed
- Zhang Y. Persistent and dormant tubercle bacilli and latent tuberculosis. Front Biosci. 2004;9:1136–1156. - PubMed
- Cooper A, Bishai WR, Flynn JA. Animal models of tuberculosis; In: Cole S, Eisenach K, Jacobs WR Jr, McMurray D, editors. Washington DC: ASM; 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous