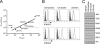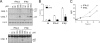Receptor density is key to the alpha2/beta interferon differential activities - PubMed (original) (raw)
Receptor density is key to the alpha2/beta interferon differential activities
Ignacio Moraga et al. Mol Cell Biol. 2009 Sep.
Abstract
Multiple type I interferons (IFN-alpha/beta) elicit Jak/Stat activation, rapid gene induction, and pleiotropic effects, such as differentiation, antiviral protection, and blocks in proliferation, which are dependent on the IFN subtype and the cellular context. To date, ligand- and receptor-specific molecular determinants underlying IFN-alpha/beta differential activities or potencies have been well characterized. To analyze cellular determinants that impact subtype-specific potency, human fibrosarcoma U5A-derived clones, exhibiting a gradient of IFN sensitivity by virtue of increasing receptor levels, were monitored for Jak/Stat signaling, gene induction, cell cycle lengthening, and apoptosis. In cells with scarce receptors, IFN-beta was more potent than IFN-alpha2 in antiproliferative activity, while the two subtypes were equipotent in all other readouts. Conversely, in cells with abundant receptors, IFN-alpha2 matched or even surpassed IFN-beta in all readouts tested. Our results suggest that the differential activities of the IFN subtypes are dictated not only by the intrinsic ligand/receptor binding kinetics but also by the density of cell surface receptor components.
Figures
FIG. 1.
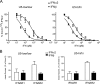
(A) Graphic representation of the relative antiproliferative potencies of IFN-α2 and IFN-β in the human cell lines 2fTGH (fibrosarcoma), WISH (amniotic), MDA231 (breast cancer), and Daudi (B lymphoma) and the three U5-derived clones (U5-low/low, U5-low/hi, and U5-hi/hi) described in this study. EC50s (pM) for IFN-α2 are plotted against the ratio of the EC50 of IFN-α2 to that of IFN-β for each cell line (Table 1). (B) Levels of IFNAR1 and IFNAR2 in the three U5-derived clones (U5-low/low, U5-low/hi, and U5-hi/hi) and in the parental 2fTGH cells. Surface IFNAR1 and IFNAR2 were quantified by FACS analysis using MAbs AA3 and CD118, respectively. Dark gray area, isotypic control; light gray area, 2fTGH; black line, U5-low/low, U5-low/hi, or U5-hi/hi. Of note, these clones exhibited similar forward scatter values, as determined by FACS analysis, indicating that the increases observed are due to changes in surface receptor density and not cell volume. (C) Analysis of the expression level of Jak/Stat pathway components in 2fTGH cells and the U5-derived clones. Total cell lysates (30 μg) were analyzed by Western blotting with the indicated Abs. Loading was evaluated by measuring Akt levels.
FIG. 2.
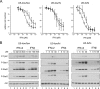
Antiproliferative and Jak/Stat activation potencies of IFN-α2 and IFN-β in U5-low/low, U5-low/hi, and U5-hi/hi cells. (A) Antiproliferative dose-response curves of U5-low/low, U5-low/hi, and U5-hi/hi cells treated for 48 h with IFN-α2 or IFN-β. Cell density was measured with crystal violet staining. Note the higher doses of IFN used for U5-low/low cells. Open circles, IFN-α2; filled circles, IFN-β. Error bars represent the standard deviation of the mean of three independent experiments, each performed in triplicate. (B) Dose-dependent profiles of tyrosine phosphorylation of Tyk2 and Stat1/2/3, measured in cells treated for 15 min with the indicated doses of IFN-α2 or IFN-β. Note that for U5-low/low cells, IFN doses ranged from 10 to 500 pM. For the other two clones, doses were from 1 pM to 30 pM. Lysates (30 μg) were analyzed by Western blotting. Loading was evaluated by measuring Akt levels. To evaluate differences, for U5-low/low cells, compare lanes 2 and 7: i.e., 10 pM IFN-α2 versus 30 pM IFN-β. For U5-low/hi cells, compare lanes 3 and 9: i.e., 5 pM IFN-α2 versus 30 pM IFN-β. For U5-hi/hi cells, compare lanes 2 and 9: i.e., 1 pM IFN-α2 versus 30 pM IFN-β. The results shown are representative of three independent experiments.
FIG. 3.
Jak/Stat activation and antiproliferative potencies of two IFN-α2 mutants on U5-low/low cells. (A) Dose-response profiles of phosphorylation of Tyk2 and Stat1/2/3 in U5-low/low cells treated for 15 min with IFN-α2, IFN-α8-tail or IFN-β (left), and IFN-α2, IFN-HEQ, or IFNβ (right). Lysates (30 μg) were analyzed by Western blotting. Loading was evaluated by measuring Akt levels. Results are representative of at least three independent experiments. (B) Antiproliferative potencies of IFN-α2, IFN-HEQ, IFN-α8-tail, and IFN-β measured on U5-low/low cells treated for 48 h. EC50s (pM) from a representative experiment are shown.
FIG. 4.
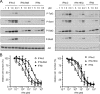
Jak/Stat activation and antiproliferative potencies of two IFN-α2 mutants on U5-hi/hi cells. (A) Dose-response profiles of phosphorylation of Tyk2 and Stat1/2/3 in U5-hi/hi cells treated for 15 min with IFN-α2, IFN-α8-tail, or IFN-β (left panels) and with IFN-α2, IFN-HEQ, or IFN-β (right panels). Lysates were analyzed as described in the legend to Fig. 3. Note that IFN-α8-tail behaved like IFN-β, and IFN-HEQ was two- to threefold more potent than IFN-β. (B) Antiproliferative dose-dependent curves of U5-hi/hi cells treated for 48 h with IFN-α2, IFN-α8-tail and IFN-β (left) or treated with IFN-α2, IFN-HEQ, and IFN-β (right). Error bars represent the standard deviation of the mean of three independent experiments, each performed in triplicate. The EC50s are 1.4 pM for IFN-α2, 6 pM for IFN-β, 45 pM for IFN-α8-tail, and 8 pM for IFN-HEQ.
FIG. 5.
Displacement of 125I-IFN-α2 and downregulation of IFNARs by IFN-α2 and IFN-β in U5-low/low and U5-hi/hi cells. (A) Displacement of 125I-labeled IFN-α2 by unlabeled IFN-α2 (open circle) or IFN-β (closed circle) was measured on U5-low/low cells (left) and U5-hi/hi (right) cells, as described in Materials and Methods. Specific cell-bound 125I-IFN-α2 was quantified and plotted. Error bars represent the standard errors of the mean of three independent experiments, each performed in triplicate. The IC50s for U5-low/low cells are 487 pM for IFN-α2 and 48 pM for IFN-β. The IC50s for U5-hi/hi cells are 209 pM for IFN-α2 and 55 pM for IFN-β. (B) IFNAR1 and IFNAR2 decay from the cell surface was measured by flow cytometry in U5-low/low (left) and U5-hi/hi (right) cells treated with IFN-α2 or IFN-β (500 pM) for 60 min in the presence of cycloheximide. Percentages of the mean fluorescence (fluores.) at time zero (mean ± standard error for at least three experiments) are shown.
FIG. 6.
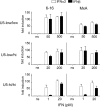
Induction of the MxA and _6_-16 genes by IFN-α2 and IFN-β in U5-low/low, U5-low/hi, and U5-hi/hi cells. Induction of the MxA gene is shown in the left panels, and induction of the _6_-16 gene is shown in the right panels in U5-low/low cells (upper), U5-low/hi cells (middle), and U5-hi/hi cells (bottom). Cells were left nonstimulated (ns) or were stimulated for 8 h with IFN-α2 (white) or IFN-β (black). Note that, for each clone, one IFN dose corresponds to the lower antiproliferative dose (EC50) and the other is 10-fold higher. Thus, U5-low/low cells (antiproliferative EC50 of IFN-β, 44 pM) were treated with 50 pM and 500 pM, U5-low/hi cells (antiproliferative EC50 of IFN-β, 18 pM) were treated with 20 and 200 pM, and U5-hi/hi cells (antiproliferative EC50 of IFN-α2, 1.4 pM) were treated with 1 and 20 pM doses. Transcripts were measured by quantitative reverse transcription-PCR; values were normalized to simultaneously quantified 18S transcripts. For each clone, the ratios between treated and nonstimulated samples are shown, taking as 1 the ratio in nonstimulated samples. Each value represents the mean of three independent experiments, each performed in triplicate.
FIG. 7.
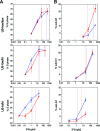
Cell cycle lengthening and apoptotic potencies of IFN-α2 and IFN-β in U5-low/low, U5-low/hi, and U5-hi/hi cells. (A) The percentage of cells (U5-low/low, U5-low/hi, and U5-hi/hi) that accumulated in S phase after 24 h of stimulation with the indicated doses of IFN-α2 (triangles) or IFN-β (circles) was measured by flow cytometry. Each value represents the mean of three independent experiments. Vertical dotted lines correspond to the antiproliferative EC50s. (B) Percentage of cells (U5-low/low, U5-low/hi, and U5-hi/hi) present in the sub-G1 phase after 48 h of stimulation with the indicated doses of IFN-α2 (triangles) or IFN-β (circles). Values were obtained from flow cytometry analysis. Each value represents the mean of three independent experiments. Vertical dotted lines correspond to the antiproliferative EC50s.
FIG. 8.
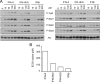
Activation of caspases and induction of Fas by IFN-α2 and IFN-β in U5-low/low cells. (A) U5-low/low cells were left untreated or treated with the indicated dose of IFN-α2 or IFN-β for 36 h. Lysates (30 μg) were analyzed by Western blotting for the presence of cleavage products of caspase (casp.) 3 (p19/p17), caspase 7 (p20), and caspase 8 (p43/p41). A nonspecific band (n.s.b) was used as the loading control. Note in the bottom panel the different order of sample loading. (B) U5-low/low cells were left untreated or were treated for 36 h with 500 pM of IFN-α2 or IFN-β in the presence or absence of the pan-caspase inhibitor Z-VAD-FMK (50 μM). The percentage of cells in sub-G1 was measured by flow cytometry. Each column represents the mean value of three independent experiments. ns, nonstimulated. (C) The surface level of Fas was monitored by flow cytometry on U5-low/low cells, untreated or treated for 4, 8, 24, and 36 h with 500 pM of IFN-α2 or IFN-β. The median fluorescence intensities (MFI) obtained in one of three independent experiments are shown.
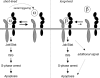
FIG. 9.
Model of receptor activation by IFN-α2 versus IFN-β and signaling outcomes The model is based on reported in vitro parameters of binary and ternary complex assembly (see references in text) and on the present cell-based analyses. The left panel shows that a short-lived ternary complex composed of IFN-α2, IFNAR2 (gray), and IFNAR1 (black) is fully fit to activate the Jak/Stat pathway, induce ISG expression, block cells in S phase, and induce a low level of apoptosis. Under the condition of a nonlimiting receptor, the rapidly dissociating ternary complexes release IFN-α2, which serially engages unoccupied receptors. This will amplify Jak/Stat activation and downstream events, leading to increased apoptosis. In other terms, in this model, the effect of IFN-α2 is not proportional to the number of occupied receptors but to the number of IFN-receptor associations per unit of time. In opposition, as illustrated in the right panel, the slowly dissociating IFN-β forms a more stable ternary complex than IFN-α2, which translates in a single quantum of Jak/Stat activation. However, it is predicted that the stable IFN-β-receptor complexes activate additional proapoptotic signals which determine the more potent antigrowth effect of IFN-β measured in cells with low-density receptors. The model could accommodate other IFN subtypes, whose competence to form a tight ternary complex may vary.
Similar articles
- Basal expression levels of IFNAR and Jak-STAT components are determinants of cell-type-specific differences in cardiac antiviral responses.
Zurney J, Howard KE, Sherry B. Zurney J, et al. J Virol. 2007 Dec;81(24):13668-80. doi: 10.1128/JVI.01172-07. Epub 2007 Oct 17. J Virol. 2007. PMID: 17942530 Free PMC article. - Virus Multiplicity of Infection Affects Type I Interferon Subtype Induction Profiles and Interferon-Stimulated Genes.
Zaritsky LA, Bedsaul JR, Zoon KC. Zaritsky LA, et al. J Virol. 2015 Nov;89(22):11534-48. doi: 10.1128/JVI.01727-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355085 Free PMC article. - The molecular basis for differential type I interferon signaling.
Schreiber G. Schreiber G. J Biol Chem. 2017 May 5;292(18):7285-7294. doi: 10.1074/jbc.R116.774562. Epub 2017 Mar 13. J Biol Chem. 2017. PMID: 28289098 Free PMC article. Review. - Alternative and accessory pathways in the regulation of IFN-beta-mediated gene expression.
Rani MR, Ransohoff RM. Rani MR, et al. J Interferon Cytokine Res. 2005 Dec;25(12):788-98. doi: 10.1089/jir.2005.25.788. J Interferon Cytokine Res. 2005. PMID: 16375607 Review.
Cited by
- Differential activity of type I interferon subtypes for dendritic cell differentiation.
Garcin G, Bordat Y, Chuchana P, Monneron D, Law HK, Piehler J, Uzé G. Garcin G, et al. PLoS One. 2013;8(3):e58465. doi: 10.1371/journal.pone.0058465. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472200 Free PMC article. - Molecular and cellular factors determining the functional pleiotropy of cytokines.
McFarlane A, Pohler E, Moraga I. McFarlane A, et al. FEBS J. 2023 May;290(10):2525-2552. doi: 10.1111/febs.16420. Epub 2022 Mar 14. FEBS J. 2023. PMID: 35246947 Free PMC article. Review. - Secretion of functional interferon by the type 3 secretion system of enteropathogenic Escherichia coli.
Rostovsky I, Wieler U, Kuzmina A, Taube R, Sal-Man N. Rostovsky I, et al. Microb Cell Fact. 2024 Jun 1;23(1):163. doi: 10.1186/s12934-024-02397-y. Microb Cell Fact. 2024. PMID: 38824527 Free PMC article. - Differences of IL-1β Receptors Expression by Immunocompetent Cells Subsets in Rheumatoid Arthritis.
Alshevskaya AA, Lopatnikova JA, Shkaruba NS, Chumasova OA, Sizikov AE, Karaulov AV, Kozlov VA, Sennikov SV. Alshevskaya AA, et al. Mediators Inflamm. 2015;2015:948393. doi: 10.1155/2015/948393. Epub 2015 Sep 10. Mediators Inflamm. 2015. PMID: 26448682 Free PMC article. - Type I interferons induce apoptosis by balancing cFLIP and caspase-8 independent of death ligands.
Apelbaum A, Yarden G, Warszawski S, Harari D, Schreiber G. Apelbaum A, et al. Mol Cell Biol. 2013 Feb;33(4):800-14. doi: 10.1128/MCB.01430-12. Epub 2012 Dec 10. Mol Cell Biol. 2013. PMID: 23230268 Free PMC article.
References
- Acuto, O., V. D. Bartolo, and F. Michel. 2008. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 8699-712. - PubMed
- Augstein, P., A. Dunger, C. Salzsieder, P. Heinke, R. Kubernath, J. Bahr, U. Fischer, R. Rettig, and E. Salzsieder. 2002. Cell surface trafficking of Fas in NIT-1 cells and dissection of surface and total Fas expression. Biochem. Biophys. Res. Commun. 290443-451. - PubMed
- Bennett, M., K. Macdonald, S. W. Chan, J. P. Luzio, R. Simari, and P. Weissberg. 1998. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 282290-293. - PubMed
- Chawla-Sarkar, M., D. J. Lindner, Y. F. Liu, B. R. Williams, G. C. Sen, R. H. Silverman, and E. C. Borden. 2003. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8237-249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources