DNA polymerase zeta cooperates with polymerases kappa and iota in translesion DNA synthesis across pyrimidine photodimers in cells from XPV patients - PubMed (original) (raw)
DNA polymerase zeta cooperates with polymerases kappa and iota in translesion DNA synthesis across pyrimidine photodimers in cells from XPV patients
Omer Ziv et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Human cells tolerate UV-induced cyclobutane pyrimidine dimers (CPD) by translesion DNA synthesis (TLS), carried out by DNA polymerase eta, the POLH gene product. A deficiency in DNA polymerase eta due to germ-line mutations in POLH causes the hereditary disease xeroderma pigmentosum variant (XPV), which is characterized by sunlight sensitivity and extreme predisposition to sunlight-induced skin cancer. XPV cells are UV hypermutable due to the activity of mutagenic TLS across CPD, which explains the cancer predisposition of the patients. However, the identity of the backup polymerase that carries out this mutagenic TLS was unclear. Here, we show that DNA polymerase zeta cooperates with DNA polymerases kappa and iota to carry out error-prone TLS across a TT CPD. Moreover, DNA polymerases zeta and kappa, but not iota, protect XPV cells against UV cytotoxicity, independently of nucleotide excision repair. This presents an extreme example of benefit-risk balance in the activity of TLS polymerases, which provide protection against UV cytotoxicity at the cost of increased mutagenic load.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
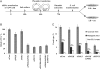
TLS across a TT CPD in XPV cells pretreated with siRNA against specific TLS polymerases. (A) Outline of the gapped plasmid TLS assay. See text for details. (B) Relative TLS extent across TT CPD lesions in XPV cells pretreated with siRNA against TLS polymerases. Each column represents the average of 6–10 measurements. See details in
Table S1
and in Materials and Methods. (C) Extent of accurate and mutagenic TLS in XPV cells pretreated with siRNA against TLS polymerases. Sequences were classified as accurate TLS (insertion of AA opposite the TT CPD), mutagenic TLS (nucleotides other than AA inserted opposite the TT CPD, or mutations at the nucleotides flanking the TT CPD), and non-TLS events (large insertions and deletions). The extent of each event type was obtained by multiplying the extent of plasmid repair (
Table S1
) by the fraction of that event obtained from the DNA sequence analysis (
Table S2
), as presented in
Table S3
.
Fig. 2.
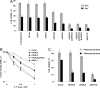
UV sensitivity of XPV and normal cells pretreated with siRNA against TLS polymerases. (A) Outline of the experimental scheme. (B and C) XPV cells (B) or normal MRC5 human cells (C) were transfected with siRNA, and UV irradiated after 48 h. For MRC5 cells, 1 mM caffeine was added immediately following UV irradiation. Viability was determined 48 h after UV irradiation by measuring cellular ATP as described in Material and Methods.
Fig. 3.
UV sensitivity of XPA cells pretreated with siRNA against TLS polymerases. Cells were transfected with siRNA, and UV irradiated after 48 h at the indicated doses. Viability was determined 48 h after UV irradiation by measuring cellular ATP (A) or 12 days after UV irradiation by measuring colony forming ability (B). (C) XPA cells stably expressing CPD photolyase were UV irradiated at 3 Jm−2 and immediately illuminated with visible light to photoreactivate CPD lesions. Cell viability was determined as in A. See Materials and Methods for details.
Fig. 4.
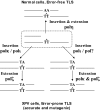
Model describing TLS across CPD in human cells. In human normal cells polη carries out efficient and relatively accurate TLS across CPD. In human XPV cells, TLS across CPD is performed by a back-up system in 2-polymerase reactions, in which polκ or polι perform insertion opposite the CPD, whereas polζ performs the extension step. This pathway is less efficient and more mutagenic than the polη-dependent pathway. A possible 3-polymerase mechanism is also presented. See text for details.
Similar articles
- Reduced efficiency and increased mutagenicity of translesion DNA synthesis across a TT cyclobutane pyrimidine dimer, but not a TT 6-4 photoproduct, in human cells lacking DNA polymerase eta.
Hendel A, Ziv O, Gueranger Q, Geacintov N, Livneh Z. Hendel A, et al. DNA Repair (Amst). 2008 Oct 1;7(10):1636-46. doi: 10.1016/j.dnarep.2008.06.008. Epub 2008 Aug 3. DNA Repair (Amst). 2008. PMID: 18634905 Free PMC article. - Evidence that in xeroderma pigmentosum variant cells, which lack DNA polymerase eta, DNA polymerase iota causes the very high frequency and unique spectrum of UV-induced mutations.
Wang Y, Woodgate R, McManus TP, Mead S, McCormick JJ, Maher VM. Wang Y, et al. Cancer Res. 2007 Apr 1;67(7):3018-26. doi: 10.1158/0008-5472.CAN-06-3073. Cancer Res. 2007. PMID: 17409408 - Complementation of defective translesion synthesis and UV light sensitivity in xeroderma pigmentosum variant cells by human and mouse DNA polymerase eta.
Yamada A, Masutani C, Iwai S, Hanaoka F. Yamada A, et al. Nucleic Acids Res. 2000 Jul 1;28(13):2473-80. doi: 10.1093/nar/28.13.2473. Nucleic Acids Res. 2000. PMID: 10871396 Free PMC article. - Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis.
Livneh Z, Ziv O, Shachar S. Livneh Z, et al. Cell Cycle. 2010 Feb 15;9(4):729-35. doi: 10.4161/cc.9.4.10727. Epub 2010 Feb 23. Cell Cycle. 2010. PMID: 20139724 Review. - The accurate bypass of pyrimidine dimers by DNA polymerase eta contributes to ultraviolet-induced mutagenesis.
Menck CFM, Galhardo RS, Quinet A. Menck CFM, et al. Mutat Res. 2024 Jan-Jun;828:111840. doi: 10.1016/j.mrfmmm.2023.111840. Epub 2023 Nov 7. Mutat Res. 2024. PMID: 37984186 Review.
Cited by
- DNA polymerase ι functions in the generation of tandem mutations during somatic hypermutation of antibody genes.
Maul RW, MacCarthy T, Frank EG, Donigan KA, McLenigan MP, Yang W, Saribasak H, Huston DE, Lange SS, Woodgate R, Gearhart PJ. Maul RW, et al. J Exp Med. 2016 Aug 22;213(9):1675-83. doi: 10.1084/jem.20151227. Epub 2016 Jul 25. J Exp Med. 2016. PMID: 27455952 Free PMC article. - A comprehensive strategy to discover inhibitors of the translesion synthesis DNA polymerase κ.
Yamanaka K, Dorjsuren D, Eoff RL, Egli M, Maloney DJ, Jadhav A, Simeonov A, Lloyd RS. Yamanaka K, et al. PLoS One. 2012;7(10):e45032. doi: 10.1371/journal.pone.0045032. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056190 Free PMC article. - Translesion synthesis of 8,5'-cyclopurine-2'-deoxynucleosides by DNA polymerases η, ι, and ζ.
You C, Swanson AL, Dai X, Yuan B, Wang J, Wang Y. You C, et al. J Biol Chem. 2013 Oct 4;288(40):28548-56. doi: 10.1074/jbc.M113.480459. Epub 2013 Aug 21. J Biol Chem. 2013. PMID: 23965998 Free PMC article. - Translesion DNA synthesis and mutagenesis in eukaryotes.
Sale JE. Sale JE. Cold Spring Harb Perspect Biol. 2013 Mar 1;5(3):a012708. doi: 10.1101/cshperspect.a012708. Cold Spring Harb Perspect Biol. 2013. PMID: 23457261 Free PMC article. Review. - A novel POLH mutation causes XP-V disease and XP-V tumor proneness may involve imbalance of numerous DNA polymerases.
Guo J, Zhou G, Zhang W, Song Y, Bian Z. Guo J, et al. Oncol Lett. 2013 Dec;6(6):1583-1590. doi: 10.3892/ol.2013.1604. Epub 2013 Oct 7. Oncol Lett. 2013. PMID: 24260050 Free PMC article.
References
- Friedberg EC, et al. DNA Repair and Mutagenesis. 2nd edition. Washington DC: ASM Press; 2006.
- Livneh Z. DNA damage control by novel DNA polymerases: Translesion replication and mutagenesis. J Biol Chem. 2001;276:25639–25642. - PubMed
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. - PubMed
- Gan GN, Wittschieben JP, Wittschieben BO, Wood RD. DNA polymerase ζ (pol ζ) in higher eukaryotes. Cell Research. 2008;18:174–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources