A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain - PubMed (original) (raw)
A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain
Marie Picot et al. J Neurosci. 2009.
Abstract
Circadian clocks synchronize to the solar day by sensing the diurnal changes in light and temperature. In adult Drosophila, the brain clock that controls rest-activity rhythms relies on neurons showing Period oscillations. Nine of these neurons are present in each larval brain hemisphere. They can receive light inputs through Cryptochrome (CRY) and the visual system, but temperature input pathways are unknown. Here, we investigate how the larval clock network responds to light and temperature. We focused on the CRY-negative dorsal neurons (DN2s), in which light-dark (LD) cycles set molecular oscillations almost in antiphase to all other clock neurons. We first showed that the phasing of the DN2s in LD depends on the pigment-dispersing factor (PDF) neuropeptide in four lateral neurons (LNs), and on the PDF receptor in the DN2s. In the absence of PDF signaling, these cells appear blind, but still synchronize to temperature cycles. Period oscillations in the DN2s were stronger in thermocycles than in LD, but with a very similar phase. Conversely, the oscillations of LNs were weaker in thermocycles than in LD, and were phase-shifted in synchrony with the DN2s, whereas the phase of the three other clock neurons was advanced by a few hours. In the absence of any other functional clock neurons, the PDF-positive LNs were entrained by LD cycles but not by temperature cycles. Our results show that the larval clock neurons respond very differently to light and temperature, and strongly suggest that the CRY-negative DN2s play a prominent role in the temperature entrainment of the network.
Figures
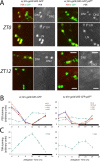
Figure 1.
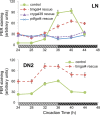
PER and TIM labeling in wild-type (left panels) and pdf01 (right panels) larval brains in LD. Larvae were entrained in LD conditions for 5 d (25°C) before dissection of 15–20 brain hemispheres for each time point. The GFP reporter expressed under tim-gal4 driver control was used to label each neuronal group. A, PER immunoreactivity in wild-type and pdf01 mutant brains at ZT0 (light on) and ZT12 (light off). Representative regions of the LNs and DNs are shown as projections of confocal stacks. In the wild type at ZT0, five LNs and two DN1s are strongly labeled, whereas the DN2s are only weakly labeled. At ZT12, no more PER labeling is visible in LNs and DN1s, whereas DN2s show stronger labeling. PER labeling in the LNs and the DN1s is unchanged in pdf01 mutants compared with wild type. In the DN2s of the mutant, however, no clear difference in labeling was observed between ZT0 and ZT12. Note also the heterogeneity in PER localization at both time points in the mutant. B, Time course of PER oscillations in different groups of larval clock neurons. Brains were dissected every 4 h during an LD cycle. The white and black boxes on _x_-axes indicate light and dark periods, respectively. PER labeling was quantified in the four PDF-positive LNs, the fifth LN, and the DN1s and DN2s, as indicated. Error bars represent the SEM for each neuronal group. Very similar results were obtained in two other experiments for each genotype. Although ANOVA found significant effects of time on PER levels in the DN2s of the pdf0 mutant, their oscillations were clearly blunted. For instance, in the experiment shown here, post hoc Dunnett's test concluded that the ZT4 time point differed only from the ZT8 time point (p < 0.05) in the mutant, while differing from ZT0 (_p_ < 0.05), ZT12, ZT16, and ZT20 (_p_ < 0.001) in the wild type. **_C_**, Time course of TIM oscillations in the larval DN2s. This was obtained as in **_B_** in an experiment in which TIM and PER were both labeled. Again, ANOVA found significant effects of time on TIM levels in the DN2s of the _pdf0_ mutant, but _post hoc_ Dunnett's test concluded that the ZT0 time point was not significantly different from any other time point (_p_ > 0.05) in the mutant, while differing from all other time points (p < 0.001) except ZT20 in the wild type.
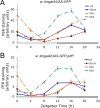
Figure 2.
Time course of molecular oscillations in the DN2s of PDF-R mutant larvae, and their rescue. Fly entrainment and PER and TIM quantification were performed as described in Figure 1. Brains were dissected every 4 h during an LD cycle. The white and black boxes on _x_-axes indicate light and dark periods, respectively. Error bars represent the SEM at each time point. A, B, PER (A) and TIM (B) oscillations in the DN2s of PDF-R mutant larvae, and in mutant larvae with PDF receptor rescue in all clock cells (tim-gal4 driver) or only the four PDF-positive LNs and the DN1s (cry-gal4–39 driver). ANOVA concluded that _cry-gal4–39_-rescued PER and TIM oscillations were not significantly different (p > 0.15) from those in the PDF-R mutant.
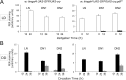
Figure 3.
PER oscillations in clock neurons of larvae with CRY-expressing DN2s, in the presence or absence of PDF. Fly entrainment and PER quantification were performed as described in Figure 1, except that all five LNs were quantified together since there was no difference between the oscillations of the four PDF-expressing LNs and those of the fifth (PDF-negative) LN. Brains were dissected at predicted peak and trough times of PER expression in LD (A) or during the 2 first days of DD (B), with CT0 corresponding to the end of the last LD cycle (when lights do not come back on). Error bars represent the SEM for each neuronal group.
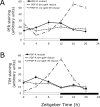
Figure 4.
Time course of PER oscillations in the larval clock neurons in temperature cycles. Wild-type (A) and pdf01 mutant (B) larvae were entrained in HC (25°C/19°C) conditions from the second day of development. Brains were dissected every 4 h during an HC cycle (ZT0 corresponds to the beginning of the hot period and ZT12 corresponds to its end). PER quantization was done as described in Figure 1. The light-gray-striped and black-striped boxes on _x_-axes indicate hot and cold periods, respectively. Error bars represent the SEM for each neuronal group.
Figure 5.
PER oscillations in the larval clock neurons in constant conditions after temperature entrainment. w;tim-gal4/UAS-gfp (control) larvae, as well as wper0 larvae with PER rescue only in the LNs (driven by pdf-gal4), or only in the LNs and DN1s (driven by cry-gal4–39), or in all clock cells (driven by tim-gal4), were entrained in HC (23°C/18°C) conditions for at least 6 d from the second day of development, and then temperature was kept constant at 18°C. Brains were dissected every 4 h during the second day in constant conditions (CT24 corresponds to the beginning of the subjective hot period and CT36 to its end). PER quantization was done as described in Figure 1. Error bars represent the SEM for each neuronal group (for the control DN2s, they are generally smaller than the symbols). Similar results were obtained at CT24–CT28 and CT36–CT40 for all genotypes in two additional experiments. ANOVA concluded that _pdf-gal4_- and _cry-gal4–39_-rescued PER variations in the LNs were not significantly different (p > 0.1) from each other, and that both were significantly different (p < 0.0001) from PER oscillations in the LNs of _tim-gal4_-rescued larvae. The light gray stripes and black stripes on medium gray background indicate subjective day and night, respectively.
Figure 6.
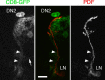
Anatomical evidence for connections between the LNs and the DN2s. Single confocal section from a w;tim-gal4/UAS-CD8-GFP;cry-gal80/+ larval brain, labeled with anti-PDF antibodies (right panel; red in middle panel). At least three GFP+ fibers can be seen along the PDF-positive ones, most clearly between the arrowheads. The arrow points to fibers that presumably run in the posterior optic tract, coming from the l-LNv precursors already detectable before metamorphosis (Helfrich-Förster et al., 2007). A GFP+ nonclock cell, which projects dorsally, is slightly out of focus. Note that, because of cry-gal80, there is no coexpression of GFP and PDF. Another sample is shown in supplemental Figure 5 (available at
as supplemental material). Scale bar, 20 μm.
Figure 7.
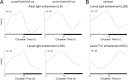
Phase of adult activity rhythms after larval entrainment by light or temperature. A, The activity profiles (waveforms) of adult flies with or without CRY-expressing DN2s were averaged over 6 d of DD and N flies, after larval (LDD) or adult (LD) entrainment by light. The light gray and black boxes indicate entrainment light and dark periods, respectively. Flies overexpressing CRY in all clock neurons (right) are compared with flies overexpressing CRY in PDF-positive LNs only (left). N, Number of flies. B, Wild-type adult activity profiles were averaged over 6 d of DD and N flies, after larval entrainment by either LD (top) or HC (bottom) cycles. Both entrainment regimens were stopped before the first pupae appeared, and replaced by constant conditions (LDD: DD at 20°C; HCC: DD at 19°C). On the _x_-axis of the top panel, the light gray and black boxes indicate entrainment light and dark periods, respectively. On the _x_-axis of the bottom panel, the boxes with light gray stripes or black stripes, on medium gray background, represent the entraining hot or cold periods, respectively.
Figure 8.
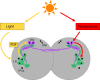
Model for larval clock responses to light and temperature cycles. In LD cycles, the larval DN2s are phased via PDF signaling from the four PDF-positive LNs (in green), which are connected to the larval visual system and express the CRY protein. In contrast, HC cycles appear to act directly on the DN2s, which oscillate more robustly than in LD, but with the same phase. The PDF-expressing LNs cycle less robustly than in LD, and with an almost opposite phase, corresponding to that of the DN2s (and differing from that of the other clock neurons). From the results of Figure 5, we propose that the LNs do not respond to HC cycles directly, but can entrain to HC only via a functional clock in the DN2s. As shown in Figure 6, fibers that appear to originate from the DN2s run parallel to the dorsal projection of the LNs, which is also in extensive contact more dorsally with the dendritic-like arborization of the DN2s. The DN2s, which are the only larval clock neurons blind to light (having neither CRY expression nor any direct visual input), thus appear to form a functional subnetwork with the LNs, which are blind to temperature, but have both CRY expression and a direct visual input.
Similar articles
- Identifying specific light inputs for each subgroup of brain clock neurons in Drosophila larvae.
Klarsfeld A, Picot M, Vias C, Chélot E, Rouyer F. Klarsfeld A, et al. J Neurosci. 2011 Nov 30;31(48):17406-15. doi: 10.1523/JNEUROSCI.5159-10.2011. J Neurosci. 2011. PMID: 22131402 Free PMC article. - Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila.
Klarsfeld A, Malpel S, Michard-Vanhée C, Picot M, Chélot E, Rouyer F. Klarsfeld A, et al. J Neurosci. 2004 Feb 11;24(6):1468-77. doi: 10.1523/JNEUROSCI.3661-03.2004. J Neurosci. 2004. PMID: 14960620 Free PMC article. - PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila.
Cusumano P, Klarsfeld A, Chélot E, Picot M, Richier B, Rouyer F. Cusumano P, et al. Nat Neurosci. 2009 Nov;12(11):1431-7. doi: 10.1038/nn.2429. Epub 2009 Oct 11. Nat Neurosci. 2009. PMID: 19820704 - A fly's eye view of circadian entrainment.
Ashmore LJ, Sehgal A. Ashmore LJ, et al. J Biol Rhythms. 2003 Jun;18(3):206-16. doi: 10.1177/0748730403018003003. J Biol Rhythms. 2003. PMID: 12828278 Review. - Control of Sleep-Wake Cycles in Drosophila.
Chatterjee A, Rouyer F. Chatterjee A, et al. 2016 Apr 5. In: Sassone-Corsi P, Christen Y, editors. A Time for Metabolism and Hormones [Internet]. Cham (CH): Springer; 2016. 2016 Apr 5. In: Sassone-Corsi P, Christen Y, editors. A Time for Metabolism and Hormones [Internet]. Cham (CH): Springer; 2016. PMID: 28892338 Free Books & Documents. Review.
Cited by
- Entrainment of the Drosophila clock by the visual system.
Schlichting M. Schlichting M. Neurosci Insights. 2020 Feb 5;15:2633105520903708. doi: 10.1177/2633105520903708. eCollection 2020. Neurosci Insights. 2020. PMID: 35174330 Free PMC article. Review. - Identifying specific light inputs for each subgroup of brain clock neurons in Drosophila larvae.
Klarsfeld A, Picot M, Vias C, Chélot E, Rouyer F. Klarsfeld A, et al. J Neurosci. 2011 Nov 30;31(48):17406-15. doi: 10.1523/JNEUROSCI.5159-10.2011. J Neurosci. 2011. PMID: 22131402 Free PMC article. - dTRPA1 Modulates Afternoon Peak of Activity of Fruit Flies Drosophila melanogaster.
Das A, Holmes TC, Sheeba V. Das A, et al. PLoS One. 2015 Jul 30;10(7):e0134213. doi: 10.1371/journal.pone.0134213. eCollection 2015. PLoS One. 2015. PMID: 26226013 Free PMC article. - Thermotaxis, circadian rhythms, and TRP channels in Drosophila.
Bellemer A. Bellemer A. Temperature (Austin). 2015 Feb 11;2(2):227-43. doi: 10.1080/23328940.2015.1004972. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227026 Free PMC article. Review. - Circadian Clock and Body Temperature.
Miyake T, Inoue Y, Maekawa Y, Doi M. Miyake T, et al. Adv Exp Med Biol. 2024;1461:177-188. doi: 10.1007/978-981-97-4584-5_12. Adv Exp Med Biol. 2024. PMID: 39289281 Review.
References
- Blanchardon E, Grima B, Klarsfeld A, Chélot E, Hardin PE, Préat T, Rouyer F. Defining the role of Drosophila lateral neurons in the control of circadian activity and eclosion rhythms by targeted genetic ablation and PERIOD protein overexpression. Eur J Neurosci. 2001;13:871–888. - PubMed
- Chang DC. Neural circuits underlying circadian behavior in Drosophila melanogaster. Behav Processes. 2006;71:211–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials