Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors - PubMed (original) (raw)
Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors
Kristofer K Rau et al. J Neurosci. 2009.
Abstract
The Mas-related G protein-coupled receptor D (Mrgprd) is selectively expressed in nonpeptidergic nociceptors that innervate the outer layers of mammalian skin. The function of Mrgprd in nociceptive neurons and the physiologically relevant somatosensory stimuli that activate Mrgprd-expressing (Mrgprd(+)) neurons are currently unknown. To address these issues, we studied three Mrgprd knock-in mouse lines using an ex vivo somatosensory preparation to examine the role of the Mrgprd receptor and Mrgprd(+) afferents in cutaneous somatosensation. In mouse hairy skin, Mrgprd, as marked by expression of green fluorescent protein reporters, was expressed predominantly in the population of nonpeptidergic, TRPV1-negative, C-polymodal nociceptors. In mice lacking Mrgprd, this population of nociceptors exhibited decreased sensitivity to cold, heat, and mechanical stimuli. Additionally, in vitro patch-clamp studies were performed on cultured dorsal root ganglion neurons from Mrgprd(-/-) and Mrgprd(+/-) mice. These studies revealed a higher rheobase in neurons from Mrgprd(-/-) mice than from Mrgprd(+/-) mice. Furthermore, the application of the Mrgprd ligand beta-alanine significantly reduced the rheobase and increased the firing rate in neurons from Mrgprd(+/-) mice but was without effect in neurons from Mrgprd(-/-) mice. Our results demonstrate that Mrgprd influences the excitability of polymodal nonpeptidergic nociceptors to mechanical and thermal stimuli.
Figures
Figure 1.
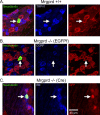
CPMs in Mrgprd knock-in and WT mice bind IB4 but do not express either TRPV1 or CGRP. A–C, Sample immunohistochemistry of recorded CPM cells in Mrgpr +/+ (A), Mrgprd −/− (EGFPf) (B), and Mrgprd −/− (Cre) (C), as indicated by biotin labeling (left panels, green). Labeling of TRPV1, CGRP, and IB4 are shown for Mrgprd–IRES–EGFPf +/+, Mrgprd −/− (EGFPf), and Mrgprd −/− (Cre), respectively (middle panels, blue). GFP labeling is shown in the right panels (red). Arrows indicate recorded cell. Scale bar, 40 μm.
Figure 2.
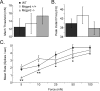
CPMs in Mrgprd −/− mice exhibit decreased mechanical sensitivity. Sharp electrode recordings were made from WT (black), Mrgprd +/+ (white), and Mrgprd −/− (gray) CPM cells that were tested for their response to mechanical stimuli. A–C, No significant difference was observed between the mice in either the average mechanical threshold (A; millinewtons) or in the mean peak instantaneous frequency (B; hertz); however, the mean rate for multiple force stimuli (C; spikes per second; 5, 10, 25, 50, and 100 mN) were decreased in the Mrgprd −/− mice. Significance (p < 0.05) is indicated below Mrgprd −/− in relation to either just WT (*) or to both WT and Mrgprd +/+ (**).
Figure 3.
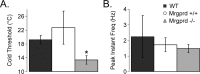
CPMs in Mrgprd −/− mice exhibit decreased cold sensitivity. Sharp electrode recordings were made from WT (black), Mrgprd +/+ (white), and Mrgprd −/− (gray) CPM cells that were tested for their response to cold stimuli. Mrgprd −/− CPM cells had lower cold thresholds (A; degrees Celsius) but maintained a similar peak instantaneous frequency (B; hertz) compared with Mrgprd +/+ mice. *p < 0.05.
Figure 4.
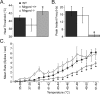
CPMs in Mrgprd −/− mice exhibit decreased heat sensitivity. Sharp electrode recordings were made from WT (black), Mrgprd +/+ (white), and Mrgprd −/− (gray) CPM cells that were tested for their response to heat stimuli. A, B, Compared with WT and Mrgprd +/+ strains, the average thermal threshold of CPMs in Mrgprd −/− mice showed decreased excitability in terms of a higher heat threshold (A; degrees Celsius) and a lower peak instantaneous frequency (B; hertz). C, A heat ramp from 31 to 52°C showed a significant reduction in the average spikes per second in Mrgprd −/− mice between the temperatures of 43 and 52°C. Significance (*p < 0.05) is indicated below Mrgprd −/− in relation to both WT and Mrgprd +/+.
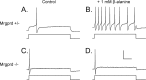
Figure 5.
β-Alanine increases the firing rate of Mrgprd +/− but not Mrgprd −/− DRG neurons. A–D, Whole-cell patch-clamp recordings were made from cultured GFP+ DRG neurons from Mrgprd +/− (A, B) and Mrgprd −/− (C, D) mice. A, C, The threshold current (1 s pulse duration; bottom trace) required to evoke one AP (top trace) was identified for each neuron. B, D, β-Alanine (1 m
m
) was then bath applied for 5 min, and the same amount of threshold current was injected into each neuron. B, Neurons expressing one functional copy of Mrgprd increased their firing rate, whereas firing rate was not increased in Mrgprd −/− neurons (D). A–D, In these cells, the threshold current was 100 pA. Calibration: 40 mV, 200 ms.
Similar articles
- Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons.
Wang H, Zylka MJ. Wang H, et al. J Neurosci. 2009 Oct 21;29(42):13202-9. doi: 10.1523/JNEUROSCI.3248-09.2009. J Neurosci. 2009. PMID: 19846708 Free PMC article. - Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain.
Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, LaMotte RH. Qu L, et al. Brain. 2014 Apr;137(Pt 4):1039-50. doi: 10.1093/brain/awu007. Epub 2014 Feb 18. Brain. 2014. PMID: 24549959 Free PMC article. - Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors.
Dussor G, Zylka MJ, Anderson DJ, McCleskey EW. Dussor G, et al. J Neurophysiol. 2008 Apr;99(4):1581-9. doi: 10.1152/jn.01396.2007. Epub 2008 Jan 30. J Neurophysiol. 2008. PMID: 18234974 Free PMC article. - Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.
St Pierre M, Reeh PW, Zimmermann K. St Pierre M, et al. Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review. - Cutaneous polymodal receptors: characteristics and plasticity.
Perl ER. Perl ER. Prog Brain Res. 1996;113:21-37. doi: 10.1016/s0079-6123(08)61079-1. Prog Brain Res. 1996. PMID: 9009726 Review.
Cited by
- Sensory neurons: An integrated component of innate immunity.
Deng L, Gillis JE, Chiu IM, Kaplan DH. Deng L, et al. Immunity. 2024 Apr 9;57(4):815-831. doi: 10.1016/j.immuni.2024.03.008. Immunity. 2024. PMID: 38599172 Review. - A mouse DRG genetic toolkit reveals morphological and physiological diversity of somatosensory neuron subtypes.
Qi L, Iskols M, Shi D, Reddy P, Walker C, Lezgiyeva K, Voisin T, Pawlak M, Kuchroo VK, Chiu IM, Ginty DD, Sharma N. Qi L, et al. Cell. 2024 Mar 14;187(6):1508-1526.e16. doi: 10.1016/j.cell.2024.02.006. Epub 2024 Mar 4. Cell. 2024. PMID: 38442711 - Schwann cells modulate nociception in neurofibromatosis 1.
Raut NG, Maile LA, Oswalt LM, Mitxelena I, Adlakha A, Sprague KL, Rupert AR, Bokros L, Hofmann MC, Patritti-Cram J, Rizvi TA, Queme LF, Choi K, Ratner N, Jankowski MP. Raut NG, et al. JCI Insight. 2024 Jan 23;9(2):e171275. doi: 10.1172/jci.insight.171275. JCI Insight. 2024. PMID: 38258905 Free PMC article. - The Mas-related G protein-coupled receptor d (Mrgprd) mediates pain hypersensitivity in painful diabetic neuropathy.
George DS, Jayaraj ND, Pacifico P, Ren D, Sriram N, Miller RE, Malfait AM, Miller RJ, Menichella DM. George DS, et al. Pain. 2024 May 1;165(5):1154-1168. doi: 10.1097/j.pain.0000000000003120. Epub 2023 Dec 22. Pain. 2024. PMID: 38147415 Free PMC article. - Pain and itch coding mechanisms of polymodal sensory neurons.
Guo C, Jiang H, Huang CC, Li F, Olson W, Yang W, Fleming M, Yu G, Hoekel G, Luo W, Liu Q. Guo C, et al. Cell Rep. 2023 Nov 28;42(11):113316. doi: 10.1016/j.celrep.2023.113316. Epub 2023 Oct 26. Cell Rep. 2023. PMID: 37889748 Free PMC article.
References
- Campagnola L, Wang H, Zylka MJ. Fiber-coupled light-emitting diode for localized photostimulation of neurons expressing channelrhodopsin-2. J Neurosci Methods. 2008;169:27–33. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS060725-02/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R56 NS023725/NS/NINDS NIH HHS/United States
- P01 NS048499/NS/NINDS NIH HHS/United States
- R01 NS052848-03/NS/NINDS NIH HHS/United States
- R01 NS023725-20/NS/NINDS NIH HHS/United States
- NS052848/NS/NINDS NIH HHS/United States
- R01 NS060725/NS/NINDS NIH HHS/United States
- R01 NS023725-21/NS/NINDS NIH HHS/United States
- R01 NS052848-04/NS/NINDS NIH HHS/United States
- R01 NS23275/NS/NINDS NIH HHS/United States
- R01 NS060725-01/NS/NINDS NIH HHS/United States
- R01 NS060725-04/NS/NINDS NIH HHS/United States
- R56 NS023725-22/NS/NINDS NIH HHS/United States
- R01 NS052848/NS/NINDS NIH HHS/United States
- R01 NS060725-03/NS/NINDS NIH HHS/United States
- R01 NS023725/NS/NINDS NIH HHS/United States
- P01 NS048499-05/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases