Taste receptors for umami: the case for multiple receptors - PubMed (original) (raw)
Taste receptors for umami: the case for multiple receptors
Nirupa Chaudhari et al. Am J Clin Nutr. 2009 Sep.
Abstract
Umami taste is elicited by many small molecules, including amino acids (glutamate and aspartate) and nucleotides (monophosphates of inosinate or guanylate, inosine 5'-monophosphate and guanosine-5'-monophosphate). Mammalian taste buds respond to these diverse compounds via membrane receptors that bind the umami tastants. Over the past 15 y, several receptors have been proposed to underlie umami detection in taste buds. These receptors include 2 glutamate-selective G protein-coupled receptors, mGluR4 and mGluR1, and the taste bud-expressed heterodimer T1R1+T1R3. Each of these receptors is expressed in small numbers of cells in anterior and posterior taste buds. The mGluRs are activated by glutamate and certain analogs but are not reported to be sensitive to nucleotides. In contrast, T1R1+T1R3 is activated by a broad range of amino acids and displays a strongly potentiated response in the presence of nucleotides. Mice in which the Grm4 gene is knocked out show a greatly enhanced preference for umami tastants. Loss of the Tas1r1 or Tas1R3 genes is reported to depress but not eliminate neural and behavioral responses to umami. When intact mammalian taste buds are apically stimulated with umami tastants, their functional responses to umami tastants do not fully resemble the responses of a single proposed umami receptor. Furthermore, the responses to umami tastants persist in the taste cells of T1R3-knockout mice. Thus, umami taste detection may involve multiple receptors expressed in different subsets of taste cells. This receptor diversity may underlie the complex perception of umami, with different mixtures of amino acids, peptides, and nucleotides yielding subtly distinct taste qualities.
Figures
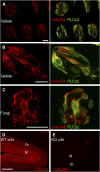
FIGURE 1
Mouse taste cells express mGluR4 and/or other group III mGluR proteins on their apical processes. A: Cryosections of vallate papilla from phospholipase C _β_2 (PLC_β_2) mice illuminated by green fluorescent protein (GFP) were incubated with anti-mGluR4 and visualized with Alexa 594-labeled secondary antibody (red). Immunoreactivity to mGluR4 and/or other group III mGluRs is seen in many cells within taste buds and appears to be limited to GFP-expressing (green) cells. B: Higher magnification of a vallate taste bud as in A, showing mGluR4-like immunofluorescence, especially in apical processes of some GFP-expressing (type II receptor) taste cells. C: mGluR4-like immunofluorescence in fungiform taste buds. Scale bars for all taste fields, 20 _μ_m. D and E: Validation of anti-mGluR4 antibody. Cryosections of cerebellum from wild-type (WT crbl) (D) and mGluR4-knockout (KO crbl) (E) mice were incubated in parallel with anti-mGluR4. Wild-type cerebellum displays a strong signal in the molecular layer (M) and a weaker signal in the granular layer (Gr), as reported previously (18), whereas knockout cerebellum shows negligible fluorescence in these layers. Scale bar, 50 _μ_m. Anti-mGluR: Rabbits were immunized with a 21-amino acid peptide, MSVRGFDRYFSSRTLDNNRRN, located near the extracellular _N_-terminus of taste-mGluR4. The same sequence is also found in full-length mGluR4 and is partially conserved in related receptors (amino acid identities of 62%, 71%, and 76% for mGluR6, mGluR7, and mGluR8, respectively). Tested in overexpressing transfected cells, the affinity-purified antibody detects full-length and truncated mGluR4 as well as mGluR8 in immunoblots and immunocytochemistry (data not shown).
Similar articles
- Multiple receptors underlie glutamate taste responses in mice.
Yasumatsu K, Horio N, Murata Y, Shirosaki S, Ohkuri T, Yoshida R, Ninomiya Y. Yasumatsu K, et al. Am J Clin Nutr. 2009 Sep;90(3):747S-752S. doi: 10.3945/ajcn.2009.27462J. Epub 2009 Jul 1. Am J Clin Nutr. 2009. PMID: 19571210 - Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice.
Yasumatsu K, Manabe T, Yoshida R, Iwatsuki K, Uneyama H, Takahashi I, Ninomiya Y. Yasumatsu K, et al. J Physiol. 2015 Feb 15;593(4):1021-34. doi: 10.1113/jphysiol.2014.284703. Epub 2015 Jan 22. J Physiol. 2015. PMID: 25529865 Free PMC article. - Metabotropic glutamate receptors are involved in the detection of IMP and L-amino acids by mouse taste sensory cells.
Pal Choudhuri S, Delay RJ, Delay ER. Pal Choudhuri S, et al. Neuroscience. 2016 Mar 1;316:94-108. doi: 10.1016/j.neuroscience.2015.12.008. Epub 2015 Dec 14. Neuroscience. 2016. PMID: 26701297 - Multiple receptor systems for glutamate detection in the taste organ.
Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. Yasuo T, et al. Biol Pharm Bull. 2008 Oct;31(10):1833-7. doi: 10.1248/bpb.31.1833. Biol Pharm Bull. 2008. PMID: 18827337 Review. - Variation in umami perception and in candidate genes for the umami receptor in mice and humans.
Shigemura N, Shirosaki S, Ohkuri T, Sanematsu K, Islam AA, Ogiwara Y, Kawai M, Yoshida R, Ninomiya Y. Shigemura N, et al. Am J Clin Nutr. 2009 Sep;90(3):764S-769S. doi: 10.3945/ajcn.2009.27462M. Epub 2009 Jul 22. Am J Clin Nutr. 2009. PMID: 19625681 Free PMC article. Review.
Cited by
- Umami taste transduction mechanisms.
Kinnamon SC. Kinnamon SC. Am J Clin Nutr. 2009 Sep;90(3):753S-755S. doi: 10.3945/ajcn.2009.27462K. Epub 2009 Jul 1. Am J Clin Nutr. 2009. PMID: 19571214 Free PMC article. Review. - Comparison of Nutritional Flavor Substances in Meat Between Shanghai Local Pig Breeds and Commercial DLY Breed.
Shi Y, Tu W, Cao M, Sun L, Zhang S, Xu J, He M, Wu C, Zhang D, Dai J, Zhou X, Gao J. Shi Y, et al. Foods. 2024 Dec 29;14(1):63. doi: 10.3390/foods14010063. Foods. 2024. PMID: 39796353 Free PMC article. - Comparing Metabolites and Functional Properties of Various Tomatoes Using Mass Spectrometry-Based Metabolomics Approach.
Mun HI, Kwon MC, Lee NR, Son SY, Song DH, Lee CH. Mun HI, et al. Front Nutr. 2021 Apr 8;8:659646. doi: 10.3389/fnut.2021.659646. eCollection 2021. Front Nutr. 2021. PMID: 33898504 Free PMC article. - Disrupted long-range gene regulations elucidate shared tissue-specific mechanisms of neuropsychiatric disorders.
Chen J, Song L, Yang A, Dong G, Zhao XM. Chen J, et al. Mol Psychiatry. 2022 Jun;27(6):2720-2730. doi: 10.1038/s41380-022-01529-3. Epub 2022 Apr 4. Mol Psychiatry. 2022. PMID: 35379909 - Biophysical and functional characterization of the N-terminal domain of the cat T1R1 umami taste receptor expressed in Escherichia coli.
Belloir C, Savistchenko J, Neiers F, Taylor AJ, McGrane S, Briand L. Belloir C, et al. PLoS One. 2017 Oct 30;12(10):e0187051. doi: 10.1371/journal.pone.0187051. eCollection 2017. PLoS One. 2017. PMID: 29084235 Free PMC article.
References
- Drake SL, Carunchia Whetstine ME, Drake MA, et al. Sources of umami taste in Cheddar and Swiss cheeses. J Food Sci 2007;72:S360–6 - PubMed
- Fuke S, Konosu S. Taste-active components in some foods: a review of Japanese research. Physiol Behav 1991;49:863–8 - PubMed
- Beksan E, Schieberle P, Robert F, et al. Synthesis and sensory characterization of novel umami-tasting glutamate glycoconjugates. J Agric Food Chem 2003;51:5428–36 - PubMed
- Barylko-Pikielna N, Kostyra E. Sensory interaction of umami substances with model food matrices and its hedonic effect. Food Qual Pref 2007;18:751–8
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous