Renal and retinal effects of enalapril and losartan in type 1 diabetes - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Jul 2;361(1):40-51.
doi: 10.1056/NEJMoa0808400.
Affiliations
- PMID: 19571282
- PMCID: PMC2978030
- DOI: 10.1056/NEJMoa0808400
Randomized Controlled Trial
Renal and retinal effects of enalapril and losartan in type 1 diabetes
Michael Mauer et al. N Engl J Med. 2009.
Abstract
Background: Nephropathy and retinopathy remain important complications of type 1 diabetes. It is unclear whether their progression is slowed by early administration of drugs that block the renin-angiotensin system.
Methods: We conducted a multicenter, controlled trial involving 285 normotensive patients with type 1 diabetes and normoalbuminuria and who were randomly assigned to receive losartan (100 mg daily), enalapril (20 mg daily), or placebo and followed for 5 years. The primary end point was a change in the fraction of glomerular volume occupied by mesangium in kidney-biopsy specimens. The retinopathy end point was a progression on a retinopathy severity scale of two steps or more. Intention-to-treat analysis was performed with the use of linear regression and logistic-regression models.
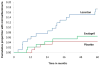
Results: A total of 90% and 82% of patients had complete renal-biopsy and retinopathy data, respectively. Change in mesangial fractional volume per glomerulus over the 5-year period did not differ significantly between the placebo group (0.016 units) and the enalapril group (0.005, P=0.38) or the losartan group (0.026, P=0.26), nor were there significant treatment benefits for other biopsy-assessed renal structural variables. The 5-year cumulative incidence of microalbuminuria was 6% in the placebo group; the incidence was higher with losartan (17%, P=0.01 by the log-rank test) but not with enalapril (4%, P=0.96 by the log-rank test). As compared with placebo, the odds of retinopathy progression by two steps or more was reduced by 65% with enalapril (odds ratio, 0.35; 95% confidence interval [CI], 0.14 to 0.85) and by 70% with losartan (odds ratio, 0.30; 95% CI, 0.12 to 0.73), independently of changes in blood pressure. There were three biopsy-related serious adverse events that completely resolved. Chronic cough occurred in 12 patients receiving enalapril, 6 receiving losartan, and 4 receiving placebo.
Conclusions: Early blockade of the renin-angiotensin system in patients with type 1 diabetes did not slow nephropathy progression but slowed the progression of retinopathy. (ClinicalTrials.gov number, NCT00143949.)
2009 Massachusetts Medical Society
Conflict of interest statement
No other potential conflicts of interest relevant to this article were disclosed.
Figures
Figure 1
Diagram of Study Patients
FIGURE 2
Kaplan-Meier Curves of Time to Microalbuminuria
Comment in
- Diabetes complications and the renin-angiotensin system.
Perkins BA, Aiello LP, Krolewski AS. Perkins BA, et al. N Engl J Med. 2009 Jul 2;361(1):83-5. doi: 10.1056/NEJMe0904293. N Engl J Med. 2009. PMID: 19571288 No abstract available. - Renal and retinal effects of enalapril and losartan in type 1 diabetes.
Tamsma JT. Tamsma JT. N Engl J Med. 2009 Oct 1;361(14):1410; author reply 1411. doi: 10.1056/NEJMc091561. N Engl J Med. 2009. PMID: 19797291 No abstract available. - Renal and retinal effects of enalapril and losartan in type 1 diabetes.
Katavetin P, Katavetin P. Katavetin P, et al. N Engl J Med. 2009 Oct 1;361(14):1410-1; author reply 1411. N Engl J Med. 2009. PMID: 19802919 No abstract available. - Early administration of enalapril and losartan to patients with type 1 diabetes.
Stanton RC. Stanton RC. Curr Diab Rep. 2009 Dec;9(6):415-6. doi: 10.1007/s11892-009-0067-9. Curr Diab Rep. 2009. PMID: 19954684 No abstract available. - Prevention of diabetic kidney disease: negative clinical trials with renin-angiotensin system inhibitors.
Nelson RG, Tuttle KR. Nelson RG, et al. Am J Kidney Dis. 2010 Mar;55(3):426-30. doi: 10.1053/j.ajkd.2009.10.001. Epub 2009 Dec 11. Am J Kidney Dis. 2010. PMID: 20005029 Free PMC article. No abstract available.
Similar articles
- ACE-I and ARBs in early diabetic nephropathy.
Mauer M, Zinman B, Gardiner R, Drummond KN, Suissa S, Donnelly SM, Strand TD, Kramer MS, Klein R, Sinaiko AR. Mauer M, et al. J Renin Angiotensin Aldosterone Syst. 2002 Dec;3(4):262-9. doi: 10.3317/jraas.2002.048. J Renin Angiotensin Aldosterone Syst. 2002. PMID: 12584670 Clinical Trial. - Renal and retinal effects of enalapril and losartan in type 1 diabetes.
Katavetin P, Katavetin P. Katavetin P, et al. N Engl J Med. 2009 Oct 1;361(14):1410-1; author reply 1411. N Engl J Med. 2009. PMID: 19802919 No abstract available. - Renal and retinal effects of enalapril and losartan in type 1 diabetes.
Tamsma JT. Tamsma JT. N Engl J Med. 2009 Oct 1;361(14):1410; author reply 1411. doi: 10.1056/NEJMc091561. N Engl J Med. 2009. PMID: 19797291 No abstract available. - Enalapril versus losartan for adults with chronic kidney disease: a systematic review and meta-analysis.
He YM, Feng L, Huo DM, Yang ZH, Liao YH. He YM, et al. Nephrology (Carlton). 2013 Sep;18(9):605-14. doi: 10.1111/nep.12134. Nephrology (Carlton). 2013. PMID: 23869492 Review. - Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review.
Sjølie AK, Dodson P, Hobbs FR. Sjølie AK, et al. Int J Clin Pract. 2011 Feb;65(2):148-53. doi: 10.1111/j.1742-1241.2010.02552.x. Int J Clin Pract. 2011. PMID: 21235695 Review.
Cited by
- Angiotensin II blockade in kidney transplant recipients.
Ibrahim HN, Jackson S, Connaire J, Matas A, Ney A, Najafian B, West A, Lentsch N, Ericksen J, Bodner J, Kasiske B, Mauer M. Ibrahim HN, et al. J Am Soc Nephrol. 2013 Feb;24(2):320-7. doi: 10.1681/ASN.2012080777. Epub 2013 Jan 10. J Am Soc Nephrol. 2013. PMID: 23308016 Free PMC article. Clinical Trial. - Prevalence and Management of Diabetic Nephropathy in Western Countries.
Satirapoj B, Adler SG. Satirapoj B, et al. Kidney Dis (Basel). 2015 May;1(1):61-70. doi: 10.1159/000382028. Epub 2015 May 1. Kidney Dis (Basel). 2015. PMID: 27536666 Free PMC article. Review. - Aldosterone antagonists in monotherapy are protective against streptozotocin-induced diabetic nephropathy in rats.
Banki NF, Ver A, Wagner LJ, Vannay A, Degrell P, Prokai A, Gellai R, Lenart L, Szakal DN, Kenesei E, Rosta K, Reusz G, Szabo AJ, Tulassay T, Baylis C, Fekete A. Banki NF, et al. PLoS One. 2012;7(6):e39938. doi: 10.1371/journal.pone.0039938. Epub 2012 Jun 28. PLoS One. 2012. PMID: 22761931 Free PMC article. - Dual blockade of Renin Angiotensin system in reducing the early changes of diabetic retinopathy and nephropathy in a diabetic rat model.
Thangaraju P, Chakrabarti A, Banerjee D, Hota D, Tamilselvan, Bhatia A, Gupta A. Thangaraju P, et al. N Am J Med Sci. 2014 Dec;6(12):625-32. doi: 10.4103/1947-2714.147978. N Am J Med Sci. 2014. PMID: 25599050 Free PMC article.
References
- Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18(10):2644–8. - PubMed
- Parving H-HMM, Ritz E. Diabetic Nephropathy. 8. Philadelphia: Saunders; 2008.
- Caramori ML, Kim Y, Huang C, et al. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes. 2002;51(2):506–13. - PubMed
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–62. - PubMed
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR000400/RR/NCRR NIH HHS/United States
- DK51975/DK/NIDDK NIH HHS/United States
- M01-RR00400/RR/NCRR NIH HHS/United States
- R01 DK051975/DK/NIDDK NIH HHS/United States
- R01 DK051975-05/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials