Autophagic control of RLR signaling - PubMed (original) (raw)
Autophagic control of RLR signaling
Michal Caspi Tal et al. Autophagy. 2009 Jul.
Abstract
Innate immunity to viral infection is initiated within the infected cells through the recognition of unique viral signatures by pattern recognition receptors (PRRs) that mediate the induction of potent antiviral factor, type I interferons (IFNs). Infection with RNA viruses is recognized by the members of the retinoic acid inducible gene I (RIG-I)-like receptor (RLR) family in the cytosol. Our recent study demonstrates that IFN production in response to RNA viral ligands is increased in the absence of autophagy. The process of autophagy functions as an internal cleanup crew within the cell, shuttling damaged cellular organelles and long-lived proteins to the lysosomes for degradation. Our data show that the absence of autophagy leads to the amplification of RLR signaling in two ways. First, in the absence of autophagy, mitochondria accumulate within the cell leading to the buildup of mitochondrial associated protein, IPS-1, a key signaling protein for RLRs. Second, damaged mitochondria that are not degraded in the absence of autophagy provide a source of reactive oxygen species (ROS), which amplify RLR signaling in Atg5 knockout cells. Our study provides the first link between ROS and cytosolic signaling mediated by the RLRs, and suggests the importance of autophagy in the regulation of signaling emanating from mitochondria.
Figures
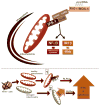
Figure 1. The mitochondria as a point of intersect between autophagy and RLR signaling
Top: a schematic representation of RLR signaling and mitochondrial clearance by autophagy. Bottom: two possibilities are illustrated for how the absence of autophagy and the resultant mitochondrial accumulation results in increased IFN and inflammatory cytokine signaling in response to RLR stimulation. First, mitochondrial accumulation results in increased levels of the mitochondrial protein IPS-1, which results in subsequent amplification of IFN and inflammatory cytokine signaling by RLRs. Second, levels of mitochondria-associated ROS rise as damaged and potentially leaky mitochondria fail to be cleared, potentiating RLR signaling.
Similar articles
- Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling.
Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Tal MC, et al. Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2770-5. doi: 10.1073/pnas.0807694106. Epub 2009 Feb 5. Proc Natl Acad Sci U S A. 2009. PMID: 19196953 Free PMC article. - Regulatory NLRs Control the RLR-Mediated Type I Interferon and Inflammatory Responses in Human Dendritic Cells.
Fekete T, Bencze D, Szabo A, Csoma E, Biro T, Bacsi A, Pazmandi K. Fekete T, et al. Front Immunol. 2018 Oct 5;9:2314. doi: 10.3389/fimmu.2018.02314. eCollection 2018. Front Immunol. 2018. PMID: 30344524 Free PMC article. - Absence of autophagy promotes apoptosis by modulating the ROS-dependent RLR signaling pathway in classical swine fever virus-infected cells.
Pei J, Deng J, Ye Z, Wang J, Gou H, Liu W, Zhao M, Liao M, Yi L, Chen J. Pei J, et al. Autophagy. 2016 Oct 2;12(10):1738-1758. doi: 10.1080/15548627.2016.1196318. Epub 2016 Jul 27. Autophagy. 2016. PMID: 27463126 Free PMC article. - RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity.
Ramos HJ, Gale M Jr. Ramos HJ, et al. Curr Opin Virol. 2011 Sep;1(3):167-76. doi: 10.1016/j.coviro.2011.04.004. Curr Opin Virol. 2011. PMID: 21949557 Free PMC article. Review. - Crosstalk between Autophagy and RLR Signaling.
Ke PY. Ke PY. Cells. 2023 Mar 21;12(6):956. doi: 10.3390/cells12060956. Cells. 2023. PMID: 36980296 Free PMC article. Review.
Cited by
- MAVS maintains mitochondrial homeostasis via autophagy.
Sun X, Sun L, Zhao Y, Li Y, Lin W, Chen D, Sun Q. Sun X, et al. Cell Discov. 2016 Aug 16;2:16024. doi: 10.1038/celldisc.2016.24. eCollection 2016. Cell Discov. 2016. PMID: 27551434 Free PMC article. - Mechanisms and Therapeutic Strategies of Viral Myocarditis Targeting Autophagy.
Yu K, Zhou L, Wang Y, Yu C, Wang Z, Liu H, Wei H, Han L, Cheng J, Wang F, Wang DW, Zhao C. Yu K, et al. Front Pharmacol. 2022 Apr 11;13:843103. doi: 10.3389/fphar.2022.843103. eCollection 2022. Front Pharmacol. 2022. PMID: 35479306 Free PMC article. Review. - Mitochondrial Interactome: A Focus on Antiviral Signaling Pathways.
Refolo G, Vescovo T, Piacentini M, Fimia GM, Ciccosanti F. Refolo G, et al. Front Cell Dev Biol. 2020 Feb 14;8:8. doi: 10.3389/fcell.2020.00008. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32117959 Free PMC article. Review. - Impact of COVID-19 on Mitochondrial-Based Immunity in Aging and Age-Related Diseases.
Ganji R, Reddy PH. Ganji R, et al. Front Aging Neurosci. 2021 Jan 12;12:614650. doi: 10.3389/fnagi.2020.614650. eCollection 2020. Front Aging Neurosci. 2021. PMID: 33510633 Free PMC article. Review. - Mechanisms of MAVS regulation at the mitochondrial membrane.
Jacobs JL, Coyne CB. Jacobs JL, et al. J Mol Biol. 2013 Dec 13;425(24):5009-19. doi: 10.1016/j.jmb.2013.10.007. Epub 2013 Oct 9. J Mol Biol. 2013. PMID: 24120683 Free PMC article. Review.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous