Molecular and cellular regulation of pancreatic acinar cell function - PubMed (original) (raw)
Review
Molecular and cellular regulation of pancreatic acinar cell function
Sohail Husain et al. Curr Opin Gastroenterol. 2009 Sep.
Abstract
Purpose of review: This review focuses on studies from the past year that have greatly advanced our understanding of molecular and cellular regulation of pancreatic acinar cell function.
Recent findings: Recent advances focus on signals dictating pancreatic development, acinar cell fate, pancreatic growth, and secretion. Regeneration of acinar cells after pancreatitis depends on expression of embryonic signals in mature acinar cells. In this setting, acinar cells can also transdifferentiate into adipose cells. With the forced induction of certain early and endocrine-driving transcription factors, acinar cells can also transdifferentiate into beta-cells. There has also been an increased understanding of acinar-to-ductal metaplasia and the subsequent formation of pancreatic intraepithelial neoplasia lesions. Multiple proteins involved in secretion have been characterized, including small guanosine triphosphate-binding proteins, soluble N-ethylmaleimide-sensitive factor attachment proteins, and ion channels.
Summary: These findings demonstrate the regenerative potential of the acinar cell to mitigate injurious states such as pancreatitis. The ability of acinar cells to transdifferentiate into beta-cells could potentially provide a treatment for diabetes. Finally, the results might be helpful in preventing malignant transformation events arising from the acinar cell. Developments in proteomics and computer modeling could expand our view of proteins mediating acinar cell function.
Figures
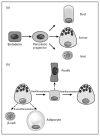
Figure 1. Developmental and differentiation patterns relating to the pancreatic acinar cell
(a) Foregut endoderm gives rise to pancreatic progenitors that then differentiate into acinar, duct, or islet cells. (b) In response to pancreatic injury, acinar cells can regenerate by dedifferentiating to a ductal epithelium and then redifferentiating to mature acinar cells (solid arrows). They can transdifferentiate to adipocytes or β-cells, depending on genetic and environmental cues (dashed arrows). However, the dedifferentiated state is prone to neoplastic transformation, such as to a PanIN lesion. PanIN, pancreatic intraepithelial neoplasia.
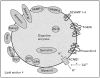
Figure 2. Zymogen granule proteins
A quantitative proteomics approach has detected previously known zymogen granule proteins (myosin V, VAMPs, syntaxins, Rab proteins and SCAMP) as well as identified new ones (Tm63A and presenilin 2). Luminal proteins include digestive enzymes as well as matrix proteins (GP2, GP3, syncollin, and ZG16). Recent advances describing the functions of some of these proteins are discussed in the text. KCNQ1, potassium voltage-gated channel, KQT-like subfamily, member 1; SCAMP, secretory carrier membrane protein; SNAP, synapse-associated protein; VAMP, vesicle-associated membrane protein.
Similar articles
- Receptor-mediated signal transduction pathways and the regulation of pancreatic acinar cell function.
Williams JA. Williams JA. Curr Opin Gastroenterol. 2008 Sep;24(5):573-9. doi: 10.1097/MOG.0b013e32830b110c. Curr Opin Gastroenterol. 2008. PMID: 19122497 Review. - Regulation of acinar cell function in the pancreas.
Williams JA. Williams JA. Curr Opin Gastroenterol. 2010 Sep;26(5):478-83. doi: 10.1097/MOG.0b013e32833d11c6. Curr Opin Gastroenterol. 2010. PMID: 20625287 Free PMC article. Review. - Regeneration and repair of the exocrine pancreas.
Murtaugh LC, Keefe MD. Murtaugh LC, et al. Annu Rev Physiol. 2015;77:229-49. doi: 10.1146/annurev-physiol-021014-071727. Epub 2014 Oct 24. Annu Rev Physiol. 2015. PMID: 25386992 Free PMC article. Review. - Murine embryonic stem cell-derived pancreatic acinar cells recapitulate features of early pancreatic differentiation.
Rovira M, Delaspre F, Massumi M, Serra SA, Valverde MA, Lloreta J, Dufresne M, Payré B, Konieczny SF, Savatier P, Real FX, Skoudy A. Rovira M, et al. Gastroenterology. 2008 Oct;135(4):1301-1310, 1310.e1-5. doi: 10.1053/j.gastro.2008.06.049. Epub 2008 Jun 25. Gastroenterology. 2008. PMID: 18725222 Free PMC article. - Interferon regulatory factor-2 regulates exocytosis mechanisms mediated by SNAREs in pancreatic acinar cells.
Mashima H, Sato T, Horie Y, Nakagawa Y, Kojima I, Ohteki T, Ohnishi H. Mashima H, et al. Gastroenterology. 2011 Sep;141(3):1102-1113.e1-8. doi: 10.1053/j.gastro.2011.05.051. Epub 2011 Jul 22. Gastroenterology. 2011. PMID: 21699790
Cited by
- Identification of KRAP-expressing cells and the functional relevance of KRAP to the subcellular localization of IP3R in the stomach and kidney.
Fujimoto T, Shirasawa S. Fujimoto T, et al. Int J Mol Med. 2012 Dec;30(6):1287-93. doi: 10.3892/ijmm.2012.1126. Epub 2012 Sep 17. Int J Mol Med. 2012. PMID: 22992961 Free PMC article. - Integrating single cell transcriptomics and volume electron microscopy confirms the presence of pancreatic acinar-like cells in sea urchins.
Paganos P, Ronchi P, Carl J, Mizzon G, Martinez P, Benvenuto G, Arnone MI. Paganos P, et al. Front Cell Dev Biol. 2022 Aug 19;10:991664. doi: 10.3389/fcell.2022.991664. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36060803 Free PMC article. - Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer.
Kang R, Xie Y, Zhang Q, Hou W, Jiang Q, Zhu S, Liu J, Zeng D, Wang H, Bartlett DL, Billiar TR, Zeh HJ 3rd, Lotze MT, Tang D. Kang R, et al. Cell Res. 2017 Jul;27(7):916-932. doi: 10.1038/cr.2017.51. Epub 2017 Apr 4. Cell Res. 2017. PMID: 28374746 Free PMC article. - Pancreatic Ubap2 deletion regulates glucose tolerance, inflammation, and protection from cerulein-induced pancreatitis.
Roy RV, Means N, Rao G, Asfa S, Madka V, Dey A, Zhang Y, Choudhury M, Fung KM, Dhanasekaran DN, Friedman JE, Crawford HC, Rao CV, Bhattacharya R, Mukherjee P. Roy RV, et al. Cancer Lett. 2023 Dec 1;578:216455. doi: 10.1016/j.canlet.2023.216455. Epub 2023 Oct 19. Cancer Lett. 2023. PMID: 37865160 Free PMC article. - Effect of Docosahexaenoic Acid on Ca2+ Signaling Pathways in Cerulein-Treated Pancreatic Acinar Cells, Determined by RNA-Sequencing Analysis.
Kim SH, Park Y, Lim JW, Kim H. Kim SH, et al. Nutrients. 2019 Jun 26;11(7):1445. doi: 10.3390/nu11071445. Nutrients. 2019. PMID: 31248019 Free PMC article.
References
- Kawaguchi Y, Cooper B, Gannon M, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. - PubMed
- Bonal C, Thorel F, Ait-Lounis A, et al. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309.e9–319.e9. This study shows that activity of the transcription factor c-Myc is required for pancreatic growth and maturation. A second intriguing finding is that pancreatic adipocyte accumulation in these mice is derived from transdifferentiated acinar cells. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK068116-05/DK/NIDDK NIH HHS/United States
- K08 DK068116-02/DK/NIDDK NIH HHS/United States
- K08 DK068116-01/DK/NIDDK NIH HHS/United States
- R03 DK078707/DK/NIDDK NIH HHS/United States
- R01 DK083327/DK/NIDDK NIH HHS/United States
- R03 DK078707-02/DK/NIDDK NIH HHS/United States
- K08 DK068116-03/DK/NIDDK NIH HHS/United States
- K08 DK068116/DK/NIDDK NIH HHS/United States
- K08 DK068116-04/DK/NIDDK NIH HHS/United States
- R03 DK078707-01A1/DK/NIDDK NIH HHS/United States
- R01 DK083327-01A1/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous