Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis - PubMed (original) (raw)
Masaru Yoshida, Makoto Arita, Yosuke Nishitani, Shin Nishiumi, Atsuhiro Masuda, Shigeto Mizuno, Tetsuya Takagawa, Yoshinori Morita, Hiromu Kutsumi, Hideto Inokuchi, Charles N Serhan, Richard S Blumberg, Takeshi Azuma
Affiliations
- PMID: 19572372
- PMCID: PMC3070396
- DOI: 10.1002/ibd.21029
Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis
Tsukasa Ishida et al. Inflamm Bowel Dis. 2010 Jan.
Abstract
Background: Resolvin E1 (RvE1), an endogenous lipid mediator derived from eicosapentaenoic acid, has been identified in local inflammation during the healing stage. RvE1 reduces inflammation in several types of animal models including peritonitis and retinopathy and blocks human neutrophil transendothelial cell migration. The RvE1 receptor ChemR23 is expressed on myeloid cells such as macrophages and dendritic cells. The aim of this study was to determine whether RvE1 regulates colonic inflammation when the innate immune response of macrophages plays a key role in pathogenesis and tissue damage.
Methods: The RvE1 receptor ChemR23 was expressed in mouse peritoneal macrophages as defined by flow cytometry. Peritoneal macrophages were pretreated with RvE1, followed by lipopolysaccharide stimulation, whereupon transcriptional levels of proinflammatory cytokines were analyzed.
Results: RvE1 treatment led to inhibition of proinflammatory cytokines including TNF-alpha and IL-12p40. In HEK293 cells, pretreatment with RvE1 inhibited TNF-alpha-induced nuclear translocation of NF-kappaB in a ChemR23-dependent manner. These results suggested that RvE1 could regulate proinflammatory responses of macrophages expressing ChemR23. Therefore, we investigated the beneficial effects of RvE1 in dextran sulfate sodium-induced colitis. RvE1 treatment led to amelioration of colonic inflammation.
Conclusions: These results indicate that RvE1 suppresses proinflammatory responses of macrophages. RvE1 and its receptor may therefore be useful as therapeutic targets in the treatment of human inflammatory bowel disease and other inflammatory disorders.
Figures
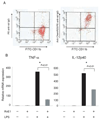
Figure 1. ChemR23 expresses on macrophages, and regulates proinflammatory cytokines
(A) Anti-mChemR23 mAb and FITC labeled anti-mCD11b mAb staining of primary mouse peritoneal macrophages. (B) Primary peritoneal macrophages were pretreated with RvE1 (100 ng/ml) for 4 hours followed by stimulation with LPS (100 ng/ml), and cytokine mRNA was analyzed by quantitative RT-PCR. Data are shown as the mean ± S.E. (n=3).
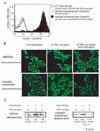
Figure 2. RvE1 inhibits the nuclear translocation of NF-κB via ChemR23
(A) Staining of Human ChemR23 stably-expressed HEK293 cells by anti-human ChemR23 mAb. (B) HEK293 wild type cells or ChemR23 transfected cells were incubated with 100 ng/ml RvE1 for 2 hours followed by treatment with 4 ng/ml TNF-α for 1 hour, and then subjected to immunocytochemistry. (C) HEK293 wild type cells or ChemR23 transfected cells were incubated with 100 ng/ml RvE1 for 2 hours followed by treatment with 4 ng/ml TNF-α for 1 hour. The nuclear proteins were prepared and analyzed for NF-κB p65 by Western blotting analysis. The band density of NF-κB was quantified, and the values relative to a vehicle control are shown. Data are shown as the mean ± S.E. (n=3).
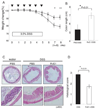
Figure 3. RvE1 dramatically attenuates DSS-induced colitis
(A) Wasting disease as measured by body weight loss in mice with DSS-induced colitis is improved by RvE1 treatment. Open circles, DSS alone; filled circles, DSS treated with RvE1 (1 µg per mouse) daily for five days (day0–5). *, P< 0.05, **, P< 0.01 (_n_=6). (B) Length of the inflamed colons of mice treated with either DSS alone or DSS plus RvE1. *P< 0.01, compared with DSS alone. (C) Colon sections of mice exposed to drinking water only (Left), day 8 after DSS-induced colitis (Center), and RvE1-treated mice (Right).(D) Histological scores of the colon of mice receiving DSS alone or DSS plus RvE1. *P<0.05, compared with DSS alone. Data are shown as the mean ± S.E. (n=6).
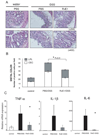
Figure 4. RvE1 reduces NF-κB phosphorylation and NF-κB-dependent proinflammatory mediators in DSS-induced colitis
(A) Immunohistchemical analysis of the effects of RvE1 on NF-κB activation (NF-κB p65 Phosphorylation at Ser276) in distal colon of mice receiving water only, DSS only, and DSS plus RvE1. Brown staining indicates cells positive for Ser276 phosphorylation of NF-κB p65. (magnification ×200: upper side, and ×400: lower side) (B) Staining was scored by counting the number of Ser276-phosphorylated NF-κB p65-positive cells (colonic epithelial cells; CEC: open bars, and lamina propria lymphocytes; LPL: shaded bars) per visualized high-power field (magnification × 400) in distal colon of mice receiving water only, DSS only, and DSS plus RvE1. (C) Quantitative real-time PCR analysis of mRNA expression of inflammatory mediators in colons obtained on day 8 from control mice (white column), mice treated with DSS alone (black column) or mice receiving DSS plus RvE1 (gray column). *, P<0.05, compared with DSS alone. Data are shown as the mean ± S.E. (n=6).
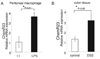
Figure 5. ChemR23 mRNA increases during LPS-stimulation and DSS induced colitis
(A) ChemR23 expression on peritoneal macrophages after 100 ng/ml LPS stimulation for 24 hours (black column) in comparison to no stimulation (white column). (B) ChemR23 expression in colon tissues of DSS induced colitis (black column) in comparison to control mice (white column). Data are shown as the mean ± S.E. (n=6).
Similar articles
- Genetic deletion of Klf4 in the mouse intestinal epithelium ameliorates dextran sodium sulfate-induced colitis by modulating the NF-κB pathway inflammatory response.
Ghaleb AM, Laroui H, Merlin D, Yang VW. Ghaleb AM, et al. Inflamm Bowel Dis. 2014 May;20(5):811-20. doi: 10.1097/MIB.0000000000000022. Inflamm Bowel Dis. 2014. PMID: 24681655 Free PMC article. - Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation.
Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Arita M, et al. J Immunol. 2007 Mar 15;178(6):3912-7. doi: 10.4049/jimmunol.178.6.3912. J Immunol. 2007. PMID: 17339491 Clinical Trial. - Resolvin E1 receptor activation signals phosphorylation and phagocytosis.
Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Ohira T, et al. J Biol Chem. 2010 Jan 29;285(5):3451-61. doi: 10.1074/jbc.M109.044131. Epub 2009 Nov 11. J Biol Chem. 2010. PMID: 19906641 Free PMC article. - The role of polyunsaturated ω-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation.
Gyurko R, Van Dyke TE. Gyurko R, et al. Crit Rev Immunol. 2014;34(4):347-57. doi: 10.1615/critrevimmunol.2014009982. Crit Rev Immunol. 2014. PMID: 24941160 Free PMC article. Review. - Function of Pro-Resolving Lipid Mediator Resolvin E1 in Type 2 Diabetes.
Sima C, Paster B, Van Dyke TE. Sima C, et al. Crit Rev Immunol. 2018;38(5):343-365. doi: 10.1615/CritRevImmunol.2018026750. Crit Rev Immunol. 2018. PMID: 30806214 Free PMC article. Review.
Cited by
- Resolution of inflammation as a novel chemopreventive strategy.
Lee HN, Na HK, Surh YJ. Lee HN, et al. Semin Immunopathol. 2013 Mar;35(2):151-61. doi: 10.1007/s00281-013-0363-y. Epub 2013 Jan 31. Semin Immunopathol. 2013. PMID: 23370700 Review. - Resolvins: natural agonists for resolution of pulmonary inflammation.
Uddin M, Levy BD. Uddin M, et al. Prog Lipid Res. 2011 Jan;50(1):75-88. doi: 10.1016/j.plipres.2010.09.002. Epub 2010 Sep 29. Prog Lipid Res. 2011. PMID: 20887750 Free PMC article. Review. - Development of a membrane-anchored chemerin receptor agonist as a novel modulator of allergic airway inflammation and neuropathic pain.
Doyle JR, Krishnaji ST, Zhu G, Xu ZZ, Heller D, Ji RR, Levy BD, Kumar K, Kopin AS. Doyle JR, et al. J Biol Chem. 2014 May 9;289(19):13385-96. doi: 10.1074/jbc.M113.522680. Epub 2014 Mar 21. J Biol Chem. 2014. PMID: 24659779 Free PMC article. - The role of macrophages and dendritic cells in the initiation of inflammation in IBD.
Steinbach EC, Plevy SE. Steinbach EC, et al. Inflamm Bowel Dis. 2014 Jan;20(1):166-75. doi: 10.1097/MIB.0b013e3182a69dca. Inflamm Bowel Dis. 2014. PMID: 23974993 Free PMC article. Review. - Resolution of Inflammation in Neurodegenerative Diseases: The Role of Resolvins.
Chamani S, Bianconi V, Tasbandi A, Pirro M, Barreto GE, Jamialahmadi T, Sahebkar A. Chamani S, et al. Mediators Inflamm. 2020 Mar 25;2020:3267172. doi: 10.1155/2020/3267172. eCollection 2020. Mediators Inflamm. 2020. PMID: 32308554 Free PMC article. Review.
References
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. - PubMed
- Belluzzi A, Brignola C, Campieri M, et al. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557–1560. - PubMed
- Teitelbaum JE, Allan Walker W. Review: the role of omega 3 fatty acids in intestinal inflammation. J Nutr Biochem. 2001;12:21–32. - PubMed
- Mayser P, Grimm H, Grimminger F. n-3 fatty acids in psoriasis. Reprod Nutr Dev. 2005;45:1–28. - PubMed
- Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71:349–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK044319/DK/NIDDK NIH HHS/United States
- DK51362/DK/NIDDK NIH HHS/United States
- DK44319/DK/NIDDK NIH HHS/United States
- R01 DK051362/DK/NIDDK NIH HHS/United States
- R01 DK053056/DK/NIDDK NIH HHS/United States
- R01 DK53056/DK/NIDDK NIH HHS/United States
- R01 DK044319-12/DK/NIDDK NIH HHS/United States
- R01 DK044319/DK/NIDDK NIH HHS/United States
- R01 DK051362-12/DK/NIDDK NIH HHS/United States
- R01 DK053056-12/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources