Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? - PubMed (original) (raw)
Review
Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier?
Maxence V Nachury et al. Annu Rev Cell Dev Biol. 2010.
Abstract
The primary cilium organizes numerous signal transduction cascades, and an understanding of signaling receptor trafficking to cilia is now emerging. A defining feature of cilia is the periciliary diffusion barrier that separates the ciliary and plasma membranes. Although lateral transport through this barrier may take place, polarized exocytosis to the base of the cilium has been the prevailing model for delivering membrane proteins to cilia. Key players for this polarized exocytosis model include the GTPases Rab8 and Rab11, the exocyst, and possibly the intraflagellar tranport machinery. In turn, the sorting of membrane proteins to cilia critically relies on the recognition of ciliary targeting signals by sorting machines such as the BBSome coat complex or the GTPase Arf4. Finally, some proteins need to exit from cilia, and ubiquitination may regulate this step. The stage is now set to dissect the interplay between signaling and regulated trafficking to and from cilia.
Figures
Figure 1
The photoreceptor cell, an exemplar of polarized trafficking to cilia. This schematic diagram of a photoreceptor cell illustrates the key steps in rhodopsin trafficking from the Golgi complex to the outer segment.
Figure 2
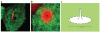
The diffusion barrier that separates ciliary and plasma membranes. (a) Glycosylphosphatidylinositol-fluorescent protein (GPI-FP, green), a GPI-anchored FP, is excluded from a zone surrounding the base of the primary cilium (acetylated tubulin, red). (b) Galectin-3 (red), a carbohydrate-binding protein, concentrates at the base of the cilium within the zone of exclusion of GPI-FP (green). (c) Schematic representation of the periciliary diffusion barrier. Images in (a) and (b) reproduced from Vieira et al. (2006), copyright © 2006, National Academy of Sciences, U.S.A.
Figure 3
Ultrastructure of the periciliary base. (a) A brick-and-mortar basal body reconstruction (Anderson 1972). The so-called transition fibers appear as wing-like structures (alar sheets) that cover most of the space at the base of the cilium. (b) Transverse electron microscopic section through the basal body transition zone (Anderson 1972). (c) Tracing of the basal body microtubule barrel and alar sheets from (b). The bottleneck shown in red can only accommodate particles 60 nm and smaller.
Figure 4
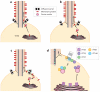
Three models for the trafficking of membrane proteins to the cilium. All models attempt to solve the problem of crossing the lipid diffusion barrier. The first two models are variations on the same theme, as both rely on polarized trafficking of post-Golgi vesicles to the base of the cilium. (a) Post-Golgi vesicles could deliver membrane proteins to the cilium by fusing with the ciliary membrane inside the ciliary shaft. (b) An extension of the previous model builds on the observation that the periciliary diffusion barrier is likely positioned at least 0.5 μm away from the base of the ciliary shaft. This 0.5-μm zone between the cilium proper and the diffusion barrier can still be considered ciliary membrane and may provide the site for vesicle docking and fusion. (c) In the last model, vesicles are delivered isotropically to the plasma membrane, and membrane proteins somehow cross the diffusion barrier (green arrow) to reach the ciliary membrane. (d) A speculative model for the sequential sorting and delivery of cargoes to cilia by intraflagellar transport (IFT) proteins. IFT20 is the only IFT protein present on the Golgi complex, and IFT20 associates with post-Golgi vesicles together with IFT52 while IFT88, -57, and -140 are present on a distinct vesicle population. En route to the base of the cilium, IFT20 is shed from the IFT complexes, and additional IFT subunits are incorporated into the cilia-destined IFT train.
Figure 5
The periciliary ridge complex (PRC) of photoreceptors. (a) Schematic cross-section of the PRC adapted from Peters et al. (1983). Note the connecting cilium (CC) at the center. (b) Scanning electron micrograph of the PRC as seen from the inner segment (Peters et al. 1983). The PRC is organized into grooves and ridges. Steps (S) are found between the deep and shallow parts inside the periciliary groove. The CC can be seen at the center of the PRC. (c) Transverse section of the PRC (Peters et al. 1983). V, vesicle. Note the central location of the CC. (d) Thin-section electron micrograph of the periciliary region 2.25 hours following radioactive amino acid labeling (Papermaster et al. 1985). Most of the label is incorporated into rhodopsin that is found in vesicles fusing in the grooves (G) of the PRC. B is the daughter centriole of the basal body complex.
Figure 6
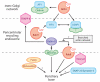
A speculative molecular pathway for membrane traffic to the primary cilium. Green peanut shapes are coats, red circles are GTPases, pink hexagons are tethering complexes, and Rabin8 (purple) is an extended coiled coil.
Figure 7
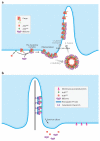
Two models for the function of the BBSome, a complex of seven highly conserved Bardet-Biedl Syndrome (BBS) proteins and one novel protein. (a) The BBSome functions as a coat complex to target membrane proteins to cilia. Upon GTP binding, Arl6 associates with membranes and recruits the BBSome. Preclustering complexes are then formed through the direct recognition of ciliary targeting signals (CTSs) by the BBSome. These BBSome/CTS/Arl6GTP complexes polymerize to form the BBSome coat and target membrane proteins to cilia. It is currently unclear whether the BBSome assembles a planar coat that mediates active lateral transport through the diffusion barrier (route 1) or a canonical coat that buds out vesicles that then fuse with the ciliary membrane (route 2). (b) The BBSome functions as an adaptor for retrograde IFT to export proteins from cilia. Note that models (a) and (b) need not be mutually exclusive.
Similar articles
- Mechanisms of ciliary targeting: entering importins and Rabs.
Lu L, Madugula V. Lu L, et al. Cell Mol Life Sci. 2018 Feb;75(4):597-606. doi: 10.1007/s00018-017-2629-3. Epub 2017 Aug 29. Cell Mol Life Sci. 2018. PMID: 28852774 Free PMC article. Review. - Loss-of-function of the ciliopathy protein Cc2d2a disorganizes the vesicle fusion machinery at the periciliary membrane and indirectly affects Rab8-trafficking in zebrafish photoreceptors.
Ojeda Naharros I, Gesemann M, Mateos JM, Barmettler G, Forbes A, Ziegler U, Neuhauss SCF, Bachmann-Gagescu R. Ojeda Naharros I, et al. PLoS Genet. 2017 Dec 27;13(12):e1007150. doi: 10.1371/journal.pgen.1007150. eCollection 2017 Dec. PLoS Genet. 2017. PMID: 29281629 Free PMC article. - A ternary complex comprising transportin1, Rab8 and the ciliary targeting signal directs proteins to ciliary membranes.
Madugula V, Lu L. Madugula V, et al. J Cell Sci. 2016 Oct 15;129(20):3922-3934. doi: 10.1242/jcs.194019. Epub 2016 Sep 15. J Cell Sci. 2016. PMID: 27633000 Free PMC article. - Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4.
Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Mazelova J, et al. EMBO J. 2009 Feb 4;28(3):183-92. doi: 10.1038/emboj.2008.267. Epub 2009 Jan 15. EMBO J. 2009. PMID: 19153612 Free PMC article. - How the Ciliary Membrane Is Organized Inside-Out to Communicate Outside-In.
Garcia G 3rd, Raleigh DR, Reiter JF. Garcia G 3rd, et al. Curr Biol. 2018 Apr 23;28(8):R421-R434. doi: 10.1016/j.cub.2018.03.010. Curr Biol. 2018. PMID: 29689227 Free PMC article. Review.
Cited by
- A Short Sequence Targets Transmembrane Proteins to Primary Cilia.
Macarelli V, Harding EC, Gershlick DC, Merkle FT. Macarelli V, et al. Cells. 2024 Jul 6;13(13):1156. doi: 10.3390/cells13131156. Cells. 2024. PMID: 38995007 Free PMC article. - Structure and dynamics of photoreceptor sensory cilia.
Wensel TG, Potter VL, Moye A, Zhang Z, Robichaux MA. Wensel TG, et al. Pflugers Arch. 2021 Sep;473(9):1517-1537. doi: 10.1007/s00424-021-02564-9. Epub 2021 May 28. Pflugers Arch. 2021. PMID: 34050409 Free PMC article. Review. - Targeting of vasoactive intestinal peptide receptor 2, VPAC2, a secretin family G-protein coupled receptor, to primary cilia.
Soetedjo L, Glover DA, Jin H. Soetedjo L, et al. Biol Open. 2013 May 23;2(7):686-94. doi: 10.1242/bio.20134747. Print 2013 Jul 15. Biol Open. 2013. PMID: 23862016 Free PMC article. Retracted. - Molecular basis for photoreceptor outer segment architecture.
Goldberg AF, Moritz OL, Williams DS. Goldberg AF, et al. Prog Retin Eye Res. 2016 Nov;55:52-81. doi: 10.1016/j.preteyeres.2016.05.003. Epub 2016 Jun 1. Prog Retin Eye Res. 2016. PMID: 27260426 Free PMC article. Review. - Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding.
Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ. Cao M, et al. Elife. 2015 Feb 17;4:e05242. doi: 10.7554/eLife.05242. Elife. 2015. PMID: 25688564 Free PMC article.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walters P. Molecular Biology of the Cell. 4th ed. Garland Sci.; New York, NY: 2002. p. 1268.
- Alieva IB, Vorobjev IA. Vertebrate primary cilia: a sensory part of centrosomal complex in tissue cells, but a “sleeping beauty” in cultured cells? Cell Biol. Int. 2004;28:139–50. - PubMed
- A very careful reconstruction of the base of the cilium shows that the so-called transition fibers are actually large sheets covering the entrance to the ciliary lumen. Ang AL, Taguchi T, Francis S, Fölsch H, Murrells LJ, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–43.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources