Individual differences in risk preference predict neural responses during financial decision-making - PubMed (original) (raw)
Individual differences in risk preference predict neural responses during financial decision-making
Jan B Engelmann et al. Brain Res. 2009.
Abstract
We investigated the neural correlates of subjective valuations during a task involving risky choices about lotteries. Because expected value was held constant across all lotteries, decisions were influenced by subjective preferences, which manifest behaviorally as risk-seeking or risk-averse attitudes. To isolate structures encoding risk preference during choice, we probed for areas showing increased activation as a function of selected risk-level. Such response patterns were obtained in anterior (ACC) and posterior cingulate cortex (PCC), superior frontal gyrus, caudate nucleus, and substantia nigra. Behavioral results revealed the presence of risk-averse and risk-neutral individuals. In parallel, brain signals revealed modulation of activity by risk attitude during choice. Correlations between risk-seeking attitudes and neural activity during risky choice were obtained in superior and inferior frontal gyri, medial and lateral orbitofrontal cortex, and parahippocampal gyrus, while correlations with risk-averse attitudes were found in the caudate. The dynamics of neural responses relevant to each stage of the task (decision, anticipation, outcome) were investigated via timeseries and conjunction analyses. Though the networks engaged in each of the task stages were mostly distinct, regions within ACC, PCC and caudate were consistently activated during each decision-making phase. These results demonstrate (1) that subjective assessments of risk, as well as individual attitudes toward risk, play a significant role in modulating activity within brain regions recruited during decision-making, and (2) that ACC, PCC and caudate are relevant during each phase of a decision-making task requiring subjective valuations, strengthening the role of these regions in self-referential subjective valuations during choice.
Figures
Figure 1
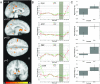
Experimental Design. (A) Schematic outline and timing of task phases. Each trial began with the presentation of a gamble pair, during which participants chose their preferred gamble, followed by an anticipation period. At the end of each trial, participants were shown the outcome of the selected trial, together with their account total. In addition, null trials followed about 24% of task trials. (B) Time course representing expected activation for each task phase. The grey areas represent the time periods during which peaks of activation for each phase are expected, based on a canonical HRF model using a gamma variate function. Hemodynamic responses are expected to peak starting about 5s after image onset during decision and outcome phases; the response peak during the anticipation period is centered between these two phases.
Figure 2
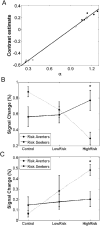
Behavior. The curvature of the utility function is determined by its α value, and represents how relatively risk-seeking (α >1), risk-neutral (α =1), or risk-averse (α <1) an individual's behavior is. Curvature of the utility function was extracted from behavioral responses of each subject using nonlinear logistic regression. Overall, the group was risk-neutral to mildly risk-seeking (median α = 1.0766, solid line), but subjects showed heterogeneous risk-related behavior, ranging from relatively risk-averse (min α = .2603, dotted line) to risk-seeking (max α = 1.2808, dashed line). The graph inset shows the curvature of the utility functions for values below 1.
Figure 3
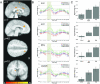
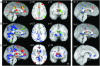
Neural correlates of risky choice during the decision phase. (A) Plots show regions with activations during the decision phase of the task that were significantly modulated by relative behavioral risk associated with a subject's choice (high-risk, low-risk, or control). Areas demonstrating this risk-related encoding include posterior cingulate (PCC), anterior cingulate (ACC), superior frontal gyrus (SFG), caudate, substantia nigra, and thalamus. For a complete list of regions showing risk-related modulation during decision-making, see Table 3. The color bar represents t values. (B) Timeseries of activations for each type of decision, high-risk (green), low-risk (blue), and control (red), for four regions, PCC, ACC, SFG (averaged across two adjacent activation clusters in BA8 and BA9) and the caudate nucleus. All regions illustrate significant risk-related modulation of neural activation intensity, with high-risk-choices eliciting the greatest response. (C) Mean peak activations in each of the regions represented in (B). In all regions, neural responses follow a significant linear trend of increasing activation with increasing relative behavioral risk.
Figure 4
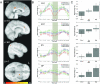
Neural correlates of risk preference. Behaviorally derived measures of individual risk preference significantly modulated the neural responses during risky choices in a network of structures that included superior frontal gyrus (SFG), inferior frontal gyrus (IFG), bilateral orbitofrontal cortex/ BA47, medial orbitofrontal cortex (mOFC), parahippocampal gyrus (PPG), and the caudate nucleus. Scatterplots on the right show individual subject's contrast estimates (betas) regressed against behavioral risk preference, α. Significant correlations between behavioral risk preference and neural activity were found in each of the illustrated areas. All correlations were positive, indicating increased BOLD activations with increased risk-seeking behavior, except for the caudate, which showed the opposite relationship.
Figure 5
Differential brain responses for risk averters and risk seekers. (A) Results from cluster analysis conducted on mean network activity and risk preference (a) for each subject. The plus signs show centroids for risk averters (bottom left) and risk seekers (top right). (B) Activity in the network of structures showing positive correlations between BOLD responses during risky choice and risk preferences (outlined in Table 4). Risk seekers exhibit an increase in activation as a function of risky choice, while risk averters show a decrease in activation. (C) Activity in the caudate nucleus shows the opposite activation pattern with a decrease as a function of risk for risk seekers and an increase for risk averters. Error bars represent standard error of the mean; “*” denotes significant differences between risk seekers and risk averters.
Figure 6
Neural correlates of risky choice during the anticipation phase: (A) Plots show regions with activations during the anticipation phase of the task that were significantly modulated by relative behavioral risk associated with subjects choices (high-risk, low-risk, or control). Regions with significant activation during the anticipation period after a risky choice included the posterior cingulate (PCC), anterior cingulate (ACC), inferior parietal lobule (IPL), and globus pallidus (GP). For a complete list of regions showing risk-related modulation during anticipation, see Table 3. (B) Timeseries of activations for each type of decision (high-risk, low-risk, or control), for four regions, PCC, ACC (averaged across two adjacent clusters in left and right hemisphere), IPL, and GP. All regions show risk-modulated neural activity that is maintained throughout the anticipation period. (C) Mean peak activations in each of the regions represented in (B). All regions illustrate a significant linear trend of increased activation with increased relative behavioral risk.
Figure 7
Outcome Phase: (A) Plots show regions significantly more active during the outcome phase for trials where the subject is informed of a win versus a loss. A diverse network of structures showed significant outcome-related modulation of activity during this phase of the task. Among these areas are included PCC, ACC, frontal eye fields (FEF), ventral striatum (vSTR), and caudate. For a complete list of regions showing outcome-related, see Table 4. (B) Timeseries of neural activity for outcomes of wins (green) and losses (red) in four regions: PCC (average of two clusters), ACC, FEF, and vSTR. In region shown here, timeseries for wins and losses maintain a similar course prior to the outcome presentation, at which point they diverge into relative increases and decreases of activation for wins and losses, respectively. (C) Mean peak activations in each of the regions represented in (B). Each region showed significantly greater BOLD responses following wins.
Figure 8
Activations across all task phases: Conjunction maps in A-C illustrate the overlap of regions significantly active (p<0.01) in each of the task phases. Though the networks are largely distinct, overlap is consistently found in regions of the ACC, PCC, and caudate nucleus, and, to a lesser extent, in thalamus, ventral striatum, cuneus, and precuneus. (A) Regions of overlap (yellow) between areas activated during decision making (red) and anticipation (green) phases. (B) Regions of overlap (turquoise) between areas activated during anticipation (green) and outcome (blue) phases. (C) Regions of overlap (pink) between areas activated during decision (red) and outcome (blue) phases. (D) Overlap remains in ACC, PCC and caudate at p < 0.001.
Similar articles
- Neural correlates of expected risks and returns in risky choice across development.
van Duijvenvoorde AC, Huizenga HM, Somerville LH, Delgado MR, Powers A, Weeda WD, Casey BJ, Weber EU, Figner B. van Duijvenvoorde AC, et al. J Neurosci. 2015 Jan 28;35(4):1549-60. doi: 10.1523/JNEUROSCI.1924-14.2015. J Neurosci. 2015. PMID: 25632132 Free PMC article. - The neural substrates of probabilistic and intertemporal decision making.
Weber BJ, Huettel SA. Weber BJ, et al. Brain Res. 2008 Oct 9;1234:104-15. doi: 10.1016/j.brainres.2008.07.105. Epub 2008 Aug 6. Brain Res. 2008. PMID: 18710652 Free PMC article. - Frontal, Striatal, and Medial Temporal Sensitivity to Value Distinguishes Risk-Taking from Risk-Aversive Older Adults during Decision Making.
Goh JO, Su YS, Tang YJ, McCarrey AC, Tereshchenko A, Elkins W, Resnick SM. Goh JO, et al. J Neurosci. 2016 Dec 7;36(49):12498-12509. doi: 10.1523/JNEUROSCI.1386-16.2016. J Neurosci. 2016. PMID: 27927964 Free PMC article. - [Risk taking and the insular cortex].
Ishii H, Tsutsui K, Iijima T. Ishii H, et al. Brain Nerve. 2013 Aug;65(8):965-72. Brain Nerve. 2013. PMID: 23917499 Review. Japanese. - A distributed, hierarchical and recurrent framework for reward-based choice.
Hunt LT, Hayden BY. Hunt LT, et al. Nat Rev Neurosci. 2017 Feb 17;18(3):172-182. doi: 10.1038/nrn.2017.7. Nat Rev Neurosci. 2017. PMID: 28209978 Free PMC article. Review.
Cited by
- Direct stimulation of anterior insula and ventromedial prefrontal cortex disrupts economic choices.
Cecchi R, Collomb-Clerc A, Rachidi I, Minotti L, Kahane P, Pessiglione M, Bastin J. Cecchi R, et al. Nat Commun. 2024 Aug 29;15(1):7508. doi: 10.1038/s41467-024-51822-8. Nat Commun. 2024. PMID: 39209840 Free PMC article. - Dynamic computational phenotyping of human cognition.
Schurr R, Reznik D, Hillman H, Bhui R, Gershman SJ. Schurr R, et al. Nat Hum Behav. 2024 May;8(5):917-931. doi: 10.1038/s41562-024-01814-x. Epub 2024 Feb 8. Nat Hum Behav. 2024. PMID: 38332340 Free PMC article. - Accentuated Paralimbic and Reduced Mesolimbic D2/3-Impulsivity Associations in Parkinson's Disease.
Stark AJ, Song AK, Petersen KJ, Hay KR, Lin YC, Trujillo P, Kang H, Collazzo JM, Donahue MJ, Zald DH, Claassen DO. Stark AJ, et al. J Neurosci. 2023 Dec 13;43(50):8733-8743. doi: 10.1523/JNEUROSCI.1037-23.2023. J Neurosci. 2023. PMID: 37852792 Free PMC article. - Risk-taking in the human brain: An activation likelihood estimation meta-analysis of the balloon analog risk task (BART).
Wang M, Zhang S, Suo T, Mao T, Wang F, Deng Y, Eickhoff S, Pan Y, Jiang C, Rao H. Wang M, et al. Hum Brain Mapp. 2022 Dec 15;43(18):5643-5657. doi: 10.1002/hbm.26041. Epub 2022 Aug 18. Hum Brain Mapp. 2022. PMID: 36441844 Free PMC article. - Sex differences in neural substrates of risk taking: Implications for sex-specific vulnerabilities to internet gaming disorder.
Wang L, Zheng H, Wang M, Chen S, Du X, Dong GH. Wang L, et al. J Behav Addict. 2022 Sep 2;11(3):778-795. doi: 10.1556/2006.2022.00057. Print 2022 Sep 26. J Behav Addict. 2022. PMID: 36053718 Free PMC article.
References
- Abdellaoui M. Parameter-free elicitation of utility and probability weighting functions. Management Science. 2000;46(11):1497–1512.
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94(1):327–37. - PubMed
- Arrow KJ. Aspects of the Theory of Risk Bearing. Yrjö Hahnsson Foundation; Helsinki: 1965.
- Barsky RB, Kimball MS, Juster FT, Shapiro MD. Preference Parameters and Behavioral Heterogeneity: An Experimental Approach in the Helath and Retirement Survey. Quarterly Journal of Economics. 1997;112:537–579.
Publication types
MeSH terms
Grants and funding
- K12 DA014040/DA/NIDA NIH HHS/United States
- T32 DA015040/DA/NIDA NIH HHS/United States
- T32 DA015040-07/DA/NIDA NIH HHS/United States
- T32 DA014040/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources