Pam17 and Tim44 act sequentially in protein import into the mitochondrial matrix - PubMed (original) (raw)
Pam17 and Tim44 act sequentially in protein import into the mitochondrial matrix
Dirk Schiller. Int J Biochem Cell Biol. 2009 Nov.
Abstract
Import of proteins into the matrix is driven by the Tim23 presequence translocase-associated import motor PAM. The core component of PAM is the mitochondrial chaperone mtHsp70, which ensures efficient translocation of proteins across the inner membrane through interactions with the J-protein complex Pam16-Pam18 (Tim16-Tim14) and its cochaperone Tim44. The recently identified non-essential Pam17 is a further member of PAM. Genetic and biochemical analyses reveal synthetic interactions between PAM17 and TIM44. Pam17 is involved in an early stage of protein translocation whereas Tim44 assists in a later step of transport, suggesting that both proteins can cooperate in a complementary manner in protein import.
Figures
Fig. 1
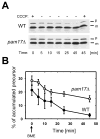
Pam17 facilitates posttranslational protein import defect in vivo. (A) Cells were grown to mid-logarithmic phase. After addition of the uncoupler CCCP incubation was continued for 30 minutes. β-mercaptoethanol was added to reverse the effect of CCCP and to restore the membrane potential across the mitochondrial inner membrane. At the times indicated, cell extracts were analyzed by immunoblotting using antibodies against the mitochondrial matrix protein Mdj1. (B) The signal intensities from four independent experiments were quantified by densitometry and the amount of preMdj1 was plotted against the incubation time using the formula: preMdj1/(preMdj1 + mMdj1). p, premature; m, mature Mdj1. BME, β-mercaptoethanol.
Fig. 2
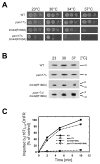
Synthetic phenotype of tim44(R180A) in the _pam17_Δ strain background. (A) Growth phenotypes of mutant strains _pam17_Δ, tim44(R180A), or _pam17_Δ/tim44(R180A). Tenfold serial dilutions were plated onto rich medium and incubated for 3 days (23°C) or 2 days (30°C, 34°C, and 37°C). (B) Accumulation of a preprotein in vivo. Cells were grown at 23°C to mid-logarithmic phase and subsequently shifted to 30°C or 37°C. Cell extracts were analyzed by immunoblotting using Hsp60-specific antibodies. p, premature Hsp60; m, mature Hsp60. (C) Import of the recombinant precursor cytochrome b2(167)Δ19-DHFR into isolated mitochondria. Kinetically saturating amounts (1 nmol/mg of mitochondrial protein) of purified preprotein was incubated with energized mitochondria for the indicated times. Following proteinase K treatment to remove unimported precursor, the amount of imported processed protein was determined by immunoblotting using antibodies against DHFR. The amount of precursor processed in wild type mitochondria after 10 minutes was set to 100% (control). The data points represent the average value of at least two independent experiments.
Fig. 3
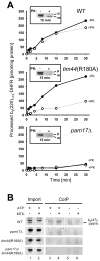
PAM17 is involved in an early stage of preprotein translocation. (A) The recombinant precursor cytochrome b2(220)Δ19-DHFR was imported into isolated mitochondria for the times indicated. Samples were split in half and treated with proteinase K (+PK) or left untreated (-PK). The amount of processed precursor in each assay was determined and plotted against the incubation time. Inset: western blot of mitochondria-associated precursor after 15 minutes of import (−PK and +PK, respectively). (B) Coimmunoprecipitation of b2(47)-DHFR as arrested precursor in mitoplasts. b2(47)-DHFR was incubated in the presence or absence of 10 μM MTX with energized mitoplasts for 10 minutes in the presence of an ATP-regenerating system (+ATP) or in the absence of ATP (-ATP). After lysis the supernatant was incubated with antibodies against Ssc1 cross linked to protein A beads. The immunoprecipitate was subjected to immunoblotting using antibodies against DHFR. p, premature b2(47)DHFR; m, mature b2(47)DHFR. The data points represent the average value of at least two independent experiments.
Fig. 4
Import of b2(47)DHFR under different conditions. (A) b2(47)-DHFR was imported into energized mitochondria: wild type (closed circles), _pam17_Δ (open circles), tim44(R180A) (closed triangles), and _pam17_Δ/tim44(R180A) (open triangles). Import of the native precursor into intact mitochondria (upper panel). The precursor was denatured by Urea-treatment prior to import (middle panel). The native precursor was imported into mitoplasts, whose outer membrane has been disrupted by hypo-osmotic swelling prior to import (lower panel). (B) Import of denatured radiolabeled b2(220)Δ19-DHFR into mitochondria. The precursor was unfolded by Urea-treatment prior to import. (C) Evaluation of the different import modes of b2(47)-DHFR into mutant mitochondria: The native (I and III) or unfolded (II) domain of DHFR (gray) and the N-terminal presequence (black rectangle) are shown. The relative import of b2(47)-DHFR as native (I and III) or denatured (II) precursor is shown. +, efficient import; -, inefficient import. OM, outer membrane, IM, inner membrane. The data points represent the average value of at least two independent experiments.
Similar articles
- Mitochondrial protein import motor: differential role of Tim44 in the recruitment of Pam17 and J-complex to the presequence translocase.
Hutu DP, Guiard B, Chacinska A, Becker D, Pfanner N, Rehling P, van der Laan M. Hutu DP, et al. Mol Biol Cell. 2008 Jun;19(6):2642-9. doi: 10.1091/mbc.e07-12-1226. Epub 2008 Apr 9. Mol Biol Cell. 2008. PMID: 18400944 Free PMC article. - Genetic analysis of complex interactions among components of the mitochondrial import motor and translocon in Saccharomyces cerevisiae.
Schilke BA, Hayashi M, Craig EA. Schilke BA, et al. Genetics. 2012 Apr;190(4):1341-53. doi: 10.1534/genetics.112.138743. Epub 2012 Jan 31. Genetics. 2012. PMID: 22298705 Free PMC article. - Pam17 is required for architecture and translocation activity of the mitochondrial protein import motor.
van der Laan M, Chacinska A, Lind M, Perschil I, Sickmann A, Meyer HE, Guiard B, Meisinger C, Pfanner N, Rehling P. van der Laan M, et al. Mol Cell Biol. 2005 Sep;25(17):7449-58. doi: 10.1128/MCB.25.17.7449-7458.2005. Mol Cell Biol. 2005. PMID: 16107694 Free PMC article. - Molecular chaperones as essential mediators of mitochondrial biogenesis.
Voos W, Röttgers K. Voos W, et al. Biochim Biophys Acta. 2002 Sep 2;1592(1):51-62. doi: 10.1016/s0167-4889(02)00264-1. Biochim Biophys Acta. 2002. PMID: 12191768 Review. - Coupling chemical energy by the hsp70/tim44 complex to drive protein translocation into mitochondria.
Cyr DM. Cyr DM. J Bioenerg Biomembr. 1997 Feb;29(1):29-34. doi: 10.1023/a:1022455621111. J Bioenerg Biomembr. 1997. PMID: 9067799 Review.
Cited by
- Sequential duplications of an ancient member of the DnaJ-family expanded the functional chaperone network in the eukaryotic cytosol.
Sahi C, Kominek J, Ziegelhoffer T, Yu HY, Baranowski M, Marszalek J, Craig EA. Sahi C, et al. Mol Biol Evol. 2013 May;30(5):985-98. doi: 10.1093/molbev/mst008. Epub 2013 Jan 16. Mol Biol Evol. 2013. PMID: 23329686 Free PMC article. - Signal recognition initiates reorganization of the presequence translocase during protein import.
Lytovchenko O, Melin J, Schulz C, Kilisch M, Hutu DP, Rehling P. Lytovchenko O, et al. EMBO J. 2013 Mar 20;32(6):886-98. doi: 10.1038/emboj.2013.23. Epub 2013 Feb 12. EMBO J. 2013. PMID: 23403928 Free PMC article. - Architecture of the TIM23 inner mitochondrial translocon and interactions with the matrix import motor.
Ting SY, Schilke BA, Hayashi M, Craig EA. Ting SY, et al. J Biol Chem. 2014 Oct 10;289(41):28689-96. doi: 10.1074/jbc.M114.588152. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25157107 Free PMC article. - Two distinct membrane potential-dependent steps drive mitochondrial matrix protein translocation.
Schendzielorz AB, Schulz C, Lytovchenko O, Clancy A, Guiard B, Ieva R, van der Laan M, Rehling P. Schendzielorz AB, et al. J Cell Biol. 2017 Jan 2;216(1):83-92. doi: 10.1083/jcb.201607066. Epub 2016 Dec 23. J Cell Biol. 2017. PMID: 28011846 Free PMC article. - Role of membrane contact sites in protein import into mitochondria.
Horvath SE, Rampelt H, Oeljeklaus S, Warscheid B, van der Laan M, Pfanner N. Horvath SE, et al. Protein Sci. 2015 Mar;24(3):277-97. doi: 10.1002/pro.2625. Epub 2015 Feb 12. Protein Sci. 2015. PMID: 25514890 Free PMC article. Review.
References
- D’Silva P, Liu Q, Walter W, Craig EA. Regulated interactions of mtHsp70 with Tim44 at the translocon in the mitochondrial inner membrane. Nat Struct Mol Biol. 2004;11:1084–91. - PubMed
- Eilers M, Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986;322:228–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous