Partial penetrance facilitates developmental evolution in bacteria - PubMed (original) (raw)
Partial penetrance facilitates developmental evolution in bacteria
Avigdor Eldar et al. Nature. 2009.
Abstract
Development normally occurs similarly in all individuals within an isogenic population, but mutations often affect the fates of individual organisms differently. This phenomenon, known as partial penetrance, has been observed in diverse developmental systems. However, it remains unclear how the underlying genetic network specifies the set of possible alternative fates and how the relative frequencies of these fates evolve. Here we identify a stochastic cell fate determination process that operates in Bacillus subtilis sporulation mutants and show how it allows genetic control of the penetrance of multiple fates. Mutations in an intercompartmental signalling process generate a set of discrete alternative fates not observed in wild-type cells, including rare formation of two viable 'twin' spores, rather than one within a single cell. By genetically modulating chromosome replication and septation, we can systematically tune the penetrance of each mutant fate. Furthermore, signalling and replication perturbations synergize to significantly increase the penetrance of twin sporulation. These results suggest a potential pathway for developmental evolution between monosporulation and twin sporulation through states of intermediate twin penetrance. Furthermore, time-lapse microscopy of twin sporulation in wild-type Clostridium oceanicum shows a strong resemblance to twin sporulation in these B. subtilis mutants. Together the results suggest that noise can facilitate developmental evolution by enabling the initial expression of discrete morphological traits at low penetrance, and allowing their stabilization by gradual adjustment of genetic parameters.
Figures
Figure 1. Partial penetrance in the developmental process of sporulation
(a) In wild-type sporulation each sporulating cell produces a single spore. (b) Partially penetrant mutants exhibit a mixture of normal sporulation, lethal failures (‘X’) and alternative viable fates (‘?’) due to cellular fluctuations (cloud). (c,d) Schematic illustrations of events (c) and genetic interactions (d) leading to differentiation of the mother cell and forespore compartments (see text).
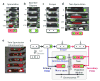
Figure 2. Time-lapse movies reveal alternative developmental pathways in spoIIRPP signaling mutants
(a-d) Green and red represent fluorescent protein expression from σF and σE-dependent promoters, respectively, overlaid on phase contrast images (gray). Developing forespores appear white at late times. Times indicated in minutes from σF activation. (a) Normal sporulation. (b) Abortively disporic cells. (c) Escaping cells activate σF but continue to elongate without activating σE (Fig. S5). Note that the activated forespore (right) does not develop further. (d) Twin sporulation occurs after escape. Green fluorescence at the initial time-point is a remnant of escape from the previous sporulation attempt. (e) Chromosome over-replication occurs prior to the formation of twin forespores. TetR-GFP-tagged chromosomal loci appear as green “dots”. Membrane staining (red) shows septation events. The rightmost dot is the remnant from a previous escape. (f) Schematic diagram showing the temporal sequence of events leading to observed terminal fates, which are classified by the numbers of chromosomes (x-axis) and compartments (y-axis). * indicates potential for return to vegetative division and/or additional sporulation attempt. Scale bar, 1μm.
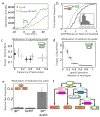
Figure 3. Noise and gene expression control cell fate in a hierarchical fashion
(a) Time traces indicating delay (arrow) and reduction (slope of yellow line compared to cyan line) in spoIIR expression rate of a typical spoIIRPP-CY cell. (b) Cumulative histograms of spoIIR expression rate are shown for two sub-populations of a single spoIIRPP strain in the same microcolony (_n_=150 cells). Sporulating cells show a systematically higher level of spoIIR expression. Inset: cell-cell variability in spoIIR expression rate. (c-e) Systematic genetic manipulation of fate penetrance. Error bars (s.e) are based on three replicate experiments. (c) spoIIR expression controls the overall frequency of sporulation (x-axis) but does not systematically affect the ratio of escape cells to abortive disporics (y-axis). Points represent spoIIRPP strains differing in spoIIR expression level and delay (supplementary methods). (d) spoIIE expression level tunes the penetrance ratio of escape to abortively disporic fates (methods, Fig. S12). (e) Deletion of yabA interacts synergistically with spoIIRPP mutants to increase twin penetrance (see also Fig. S11). (f) Fate determination can be controlled hierarchically—different genes affect different decision points.
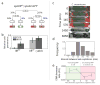
Figure 4. Evolution of twin sporulation
(a) Fate tree showing relative frequencies of over-replication (second row) and additional septation (third row) inferred from analysis of terminal fates (bottom row) of _n=_285 individual cells. Note that the probability of having three compartments depends on chromosome number (blue percentages). Day to day variation was ≤ 2% across all measurements. (b) Strain backgrounds PY79 (used throughout the paper) and BR151 differ in twin penetrance with the same spoIIRPP mutation (error bars, s.e., based on multiple experiments). yabA mutations reduce this difference. (c-e) Twin sporulation in C. oceanicum resembles that in B. subtilis mutants. (c) Filmstrip shows typical events during C. oceanicum sporulation (times in minutes from first frame). Shown are DNA (green), membrane staining (red), and phase contrast (gray). Yellow arrowheads mark first appearance of asymmetric septa. (d) The distribution of time intervals between two septation events during twin sporulation (_n_=70). (e) The rate of change of DNA staining was quantified in individual cells. Staining increases prior to septation (green area), consistent with chromosome replication, and decreases after septation (red area), consistent with transport of DNA into forespores. Data were averaged over _n_=30 cells due to cell-cell variability (error bars, s.e.m.).
Similar articles
- Hierarchical evolution of the bacterial sporulation network.
de Hoon MJ, Eichenberger P, Vitkup D. de Hoon MJ, et al. Curr Biol. 2010 Sep 14;20(17):R735-45. doi: 10.1016/j.cub.2010.06.031. Curr Biol. 2010. PMID: 20833318 Free PMC article. Review. - Hpr (ScoC) and the phosphorelay couple cell cycle and sporulation in Bacillus subtilis.
Shafikhani SH, Núñez E, Leighton T. Shafikhani SH, et al. FEMS Microbiol Lett. 2004 Feb 9;231(1):99-110. doi: 10.1016/S0378-1097(03)00936-4. FEMS Microbiol Lett. 2004. PMID: 14769473 - Functional requirements of cellular differentiation: lessons from Bacillus subtilis.
Narula J, Fujita M, Igoshin OA. Narula J, et al. Curr Opin Microbiol. 2016 Dec;34:38-46. doi: 10.1016/j.mib.2016.07.011. Epub 2016 Aug 6. Curr Opin Microbiol. 2016. PMID: 27501460 Review. - Heterochronic phosphorelay gene expression as a source of heterogeneity in Bacillus subtilis spore formation.
de Jong IG, Veening JW, Kuipers OP. de Jong IG, et al. J Bacteriol. 2010 Apr;192(8):2053-67. doi: 10.1128/JB.01484-09. Epub 2010 Feb 12. J Bacteriol. 2010. PMID: 20154131 Free PMC article. - Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis.
Gholamhoseinian A, Piggot PJ. Gholamhoseinian A, et al. J Bacteriol. 1989 Oct;171(10):5747-9. doi: 10.1128/jb.171.10.5747-5749.1989. J Bacteriol. 1989. PMID: 2507532 Free PMC article.
Cited by
- 6''-Thioether tobramycin analogues: towards selective targeting of bacterial membranes.
Herzog IM, Green KD, Berkov-Zrihen Y, Feldman M, Vidavski RR, Eldar-Boock A, Satchi-Fainaro R, Eldar A, Garneau-Tsodikova S, Fridman M. Herzog IM, et al. Angew Chem Int Ed Engl. 2012 Jun 4;51(23):5652-6. doi: 10.1002/anie.201200761. Epub 2012 Apr 12. Angew Chem Int Ed Engl. 2012. PMID: 22499286 Free PMC article. No abstract available. - Bacterial polarity.
Bowman GR, Lyuksyutova AI, Shapiro L. Bowman GR, et al. Curr Opin Cell Biol. 2011 Feb;23(1):71-7. doi: 10.1016/j.ceb.2010.10.013. Epub 2010 Nov 20. Curr Opin Cell Biol. 2011. PMID: 21095111 Free PMC article. - Molecular Time Sharing through Dynamic Pulsing in Single Cells.
Park J, Dies M, Lin Y, Hormoz S, Smith-Unna SE, Quinodoz S, Hernández-Jiménez MJ, Garcia-Ojalvo J, Locke JCW, Elowitz MB. Park J, et al. Cell Syst. 2018 Feb 28;6(2):216-229.e15. doi: 10.1016/j.cels.2018.01.011. Epub 2018 Feb 14. Cell Syst. 2018. PMID: 29454936 Free PMC article. - New insights into the genetics of in vivo induction of maternal haploids, the backbone of doubled haploid technology in maize.
Prigge V, Xu X, Li L, Babu R, Chen S, Atlin GN, Melchinger AE. Prigge V, et al. Genetics. 2012 Feb;190(2):781-93. doi: 10.1534/genetics.111.133066. Epub 2011 Nov 30. Genetics. 2012. PMID: 22135357 Free PMC article. - History-dependent physiological adaptation to lethal genetic modification under antibiotic exposure.
Koganezawa Y, Umetani M, Sato M, Wakamoto Y. Koganezawa Y, et al. Elife. 2022 May 10;11:e74486. doi: 10.7554/eLife.74486. Elife. 2022. PMID: 35535492 Free PMC article.
References
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–24. - PubMed
- Coote JG. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J Gen Microbiol. 1972;71:1–15. - PubMed
- Rutherford SL, Henikoff S. Quantitative epigenetics. Nat Genet. 2003;33:6–8. - PubMed
Publication types
MeSH terms
Grants and funding
- P50 GM068763/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 GM043577/GM/NIGMS NIH HHS/United States
- R01GM079771/GM/NIGMS NIH HHS/United States
- R01 GM079771/GM/NIGMS NIH HHS/United States
- GM43577/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources