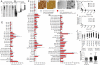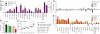Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer's disease - PubMed (original) (raw)
. 2009 Jul 21;106(29):12145-50.
doi: 10.1073/pnas.0904866106. Epub 2009 Jul 6.
C E Olin, H T Johns, Y Takeda-Uchimura, M C LeMieux, K Rufibach, J Rajadas, H Zhang, B Tomooka, W H Robinson, C M Clark, A M Fagan, D R Galasko, D M Holtzman, M Jutel, J A Kaye, C A Lemere, J Leszek, G Li, E R Peskind, J F Quinn, J A Yesavage, J A Ghiso, T Wyss-Coray
Affiliations
- PMID: 19581601
- PMCID: PMC2715538
- DOI: 10.1073/pnas.0904866106
Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer's disease
M Britschgi et al. Proc Natl Acad Sci U S A. 2009.
Abstract
A number of distinct beta-amyloid (Abeta) variants or multimers have been implicated in Alzheimer's disease (AD), and antibodies recognizing such peptides are in clinical trials. Humans have natural Abeta-specific antibodies, but their diversity, abundance, and function in the general population remain largely unknown. Here, we demonstrate with peptide microarrays the presence of natural antibodies against known toxic Abeta and amyloidogenic non-Abeta species in plasma samples and cerebrospinal fluid of AD patients and healthy controls aged 21-89 years. Antibody reactivity was most prominent against oligomeric assemblies of Abeta and pyroglutamate or oxidized residues, and IgGs specific for oligomeric preparations of Abeta1-42 in particular declined with age and advancing AD. Most individuals showed unexpected antibody reactivities against peptides unique to autosomal dominant forms of dementia (mutant Abeta, ABri, ADan) and IgGs isolated from plasma of AD patients or healthy controls protected primary neurons from Abeta toxicity. Aged vervets showed similar patterns of plasma IgG antibodies against amyloid peptides, and after immunization with Abeta the monkeys developed high titers not only against Abeta peptides but also against ABri and ADan peptides. Our findings support the concept of conformation-specific, cross-reactive antibodies that may protect against amyloidogenic toxic peptides. If a therapeutic benefit of Abeta antibodies can be confirmed in AD patients, stimulating the production of such neuroprotective antibodies or passively administering them to the elderly population may provide a preventive measure toward AD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Human plasma IgGs preferentially recognize oligomeric and postranslationally modified Aβ. (A) Membranes with fibrillar (f) and oligomeric (o) preparations of 2 μg of Aβ1-42 were probed with biotinylated human IgG fractions isolated from plasma of 2 NDCs and 2 AD patients. Bound IgGs were detected by HRP-tagged avidin. (B) In parallel experiments membranes were probed with Aβ1–5-specific mAb 3D6 (0.5 μg/mL). Because 3D6 did not detect the high molecular mass species of Aβ1-42 in the oligomeric preparation after the first probing, the lane with the fibrillar preparation was cut off and the truncated membrane strip containing oligomeric Aβ1-42 >17 kDa was reprobed with fresh antibody. This was repeated 1 more time after cutting the strip >55 kDa. (C and D) Fibrillar (C) and oligomeric (D) preparations of Aβ1-42 analyzed by atomic force microscopy (5-nm total _z_-range). (E–G) Human IgG reactivities against peptide preparations were measured with antigen microarrays in plasma from AD patients and NDC in sample set 1 (E) and sample set 2 (F and G). Bars represent mean ± SEM. Names underlined with dashed lines are synthetic mutant peptides not present in vivo. No significant differences in antibody reactivities were observed between samples from AD patients and NDC for any antigen in both sample sets (SAM). (H and I) Titration of plasma IgG (H) and IgM (I) reactivity to oligomeric Aβ1-42 in 3 samples. (J and K) Antibody reactivities against groups of peptides (closed symbols) compared with reactivities against control antigens (open symbols) in sample set 2. Each dot represents median measurement for 1 plasma sample (log scale; mean reactivity; ***, P ≤ 0.001; Mann–Whitney U test or Kruskal-Wallis 1-way ANOVA followed by Dunn's post hoc test.) (L and M) Electron microscopy analysis of assembly status of nonaggregated (L) and oligomeric (M) preparation of Bri16–23(A16K/V17K). DFU, digital fluorescent units.
Fig. 2.
Reactivity to oligomeric assemblies of Aβ peptides decline with progression of AD and together with other reactivities also decrease with age. (A and B) Average antibody reactivity for Aβ1-42 oligomer preparations in AD patients with mild (MMSE score ≥20, orange dots) or moderate to severe stages (MMSE score ≤19, red dots) of the disease in sample set 1 (A) and sample set 2 (B) (mean reactivity; **, P ≤ 0.01, Mann–Whitney U test). Each dot represents median measurement for 1 plasma sample. (C) Unsupervised clustering of antibody reactivities that are significantly associated with age in healthy females (n = 66) ages 21–85 years (SAM). Age of donors is indicated on top of the node map as boxes with increasing intensities of purple for higher age. Color shades in node map indicate higher (red) or lower (blue) antibody reactivity. (D) ELISA measurements for free (untreated) and total (immune complex dissociation at pH 3.5) antibody reactivity against oligomeric species of Aβ1-42 in randomly selected plasma samples of healthy individuals from different age groups (n = 11–15 samples per group) of sample set 3 and AD patients (n = 13) of sample set 2 (mean; 1-way ANOVA). DFU, digital fluorescent units; OD450, optical density at 450 nm.
Fig. 3.
IgGs to Aβ are present in human CSF, protect neurons from Aβ toxicity in vitro, and are amplified together with ABri and ADan cross-reactive antibodies in an Aβ immunization paradigm. (A) IgG reactivity pattern in CSF of AD patients (≥70 years) and NDC young (24–48 years) and aged individuals (58–83 years). Bars represent means of median reactivities ± SEM. (B and C) Protective effect of human IgGs on primary mouse E16 neurons incubated with oligomeric preparation of Aβ1-42 was measured by percentage LDH release in dying cells (B) and percentage generation of formazan (C). Amount of water-soluble formazan produced by surviving cells in culture medium alone was considered 100% survival. Bars represent mean ± SEM of 3 experiments done in triplicates. Symbols in scatter plot represent mean of 1 triplicate experiment. *, P ≤ 0.05, 1-way ANOVA and Dunnett's multiple comparison test with “no antibody” as control. (D and E) Vervet monkeys were left untreated (D) or immunized with a mixture of Aβ1-40 and Aβ1-42 (E) (39). Immunoreactivity at the end of the study (day 301) was divided by the baseline reactivity to individual peptides, and the result is presented as log fold change. Bars represent median fold change of reactivity to a peptide for each individual animal. As expected, immunoreactivity to Aβ1-40 and Aβ1-42 peptides and oligomeric assemblies increase over several log ranges. Note the increase in reactivity to Aβ11(pE)-42, ABri, and ADan but the lack of increase to Aβ33–42. DFU, digital fluorescent units.
Similar articles
- Chemical traits of cerebral amyloid angiopathy in familial British-, Danish-, and non-Alzheimer's dementias.
Michno W, Koutarapu S, Camacho R, Toomey C, Stringer K, Minta K, Ge J, Jha D, Fernandez-Rodriguez J, Brinkmalm G, Zetterberg H, Blennow K, Ryan NS, Lashley T, Hanrieder J. Michno W, et al. J Neurochem. 2022 Nov;163(3):233-246. doi: 10.1111/jnc.15694. Epub 2022 Sep 25. J Neurochem. 2022. PMID: 36102248 Free PMC article. - A cyclic undecamer peptide mimics a turn in folded Alzheimer amyloid β and elicits antibodies against oligomeric and fibrillar amyloid and plaques.
Hoogerhout P, Kamphuis W, Brugghe HF, Sluijs JA, Timmermans HA, Westdijk J, Zomer G, Boog CJ, Hol EM, van den Dobbelsteen GP. Hoogerhout P, et al. PLoS One. 2011 Apr 19;6(4):e19110. doi: 10.1371/journal.pone.0019110. PLoS One. 2011. PMID: 21526148 Free PMC article. - Human intravenous immunoglobulin provides protection against Aβ toxicity by multiple mechanisms in a mouse model of Alzheimer's disease.
Magga J, Puli L, Pihlaja R, Kanninen K, Neulamaa S, Malm T, Härtig W, Grosche J, Goldsteins G, Tanila H, Koistinaho J, Koistinaho M. Magga J, et al. J Neuroinflammation. 2010 Dec 7;7:90. doi: 10.1186/1742-2094-7-90. J Neuroinflammation. 2010. PMID: 21138577 Free PMC article. - Developing novel immunogens for a safe and effective Alzheimer's disease vaccine.
Lemere CA. Lemere CA. Prog Brain Res. 2009;175:83-93. doi: 10.1016/S0079-6123(09)17506-4. Prog Brain Res. 2009. PMID: 19660650 Free PMC article. Review. - Immunotherapy for Alzheimer's disease: from anti-β-amyloid to tau-based immunization strategies.
Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, Greco A, Seripa D, Pilotto A. Panza F, et al. Immunotherapy. 2012 Feb;4(2):213-38. doi: 10.2217/imt.11.170. Immunotherapy. 2012. PMID: 22339463 Review.
Cited by
- Integrated bioinformatics-based identification of diagnostic markers in Alzheimer disease.
Chen D, Zhang Y, Qiao R, Kong X, Zhong H, Wang X, Zhu J, Li B. Chen D, et al. Front Aging Neurosci. 2022 Nov 10;14:988143. doi: 10.3389/fnagi.2022.988143. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36437991 Free PMC article. - Naturally occurring autoantibodies interfere with β-amyloid metabolism and improve cognition in a transgenic mouse model of Alzheimer's disease 24 h after single treatment.
Mengel D, Röskam S, Neff F, Balakrishnan K, Deuster O, Gold M, Oertel WH, Bacher M, Bach JP, Dodel R. Mengel D, et al. Transl Psychiatry. 2013 Mar 5;3(3):e236. doi: 10.1038/tp.2012.151. Transl Psychiatry. 2013. PMID: 23462987 Free PMC article. - A lipoprotein receptor cluster IV mutant preferentially binds amyloid-β and regulates its clearance from the mouse brain.
Sagare AP, Bell RD, Srivastava A, Sengillo JD, Singh I, Nishida Y, Chow N, Zlokovic BV. Sagare AP, et al. J Biol Chem. 2013 May 24;288(21):15154-66. doi: 10.1074/jbc.M112.439570. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580652 Free PMC article. - Altered levels of circulating natural antibodies against VEGFR1-derived peptide in atherosclerosis.
Wang P, Liu S, Wang Z, Zhao H, Zhang X. Wang P, et al. J Int Med Res. 2020 Aug;48(8):300060520948750. doi: 10.1177/0300060520948750. J Int Med Res. 2020. PMID: 32811267 Free PMC article. - Is Alzheimer disease a failure of mobilizing immune defense? Lessons from cognitively fit oldest-old.
Katsel P, Haroutunian V. Katsel P, et al. Dialogues Clin Neurosci. 2019 Mar;21(1):7-19. doi: 10.31887/DCNS.2019.21.1/vharoutunian. Dialogues Clin Neurosci. 2019. PMID: 31607776 Free PMC article.
References
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
- Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid β protein in Alzheimer's disease. J Biol Chem. 1992;267:17082–17086. - PubMed
- Saido TC, et al. Dominant and differential deposition of distinct β-amyloid peptide species, A β N3(pE), in senile plaques. Neuron. 1995;14:457–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG030539/AG/NIA NIH HHS/United States
- AG10491/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- R01 AG020603/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P01 AG010491/AG/NIA NIH HHS/United States
- R01 AG030539/AG/NIA NIH HHS/United States
- AG20603/AG/NIA NIH HHS/United States
- AG08017/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases