Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy - PubMed (original) (raw)
Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy
Wen-Xing Ding et al. Mol Cancer Ther. 2009 Jul.
Abstract
The proteasome and the autophagy systems are two evolutionarily conserved mechanisms for degrading intracellular materials. They are functionally coupled and suppression of the proteasome promotes autophagy. Although suppression of the proteasome leads to cell death, suppression of autophagy can be either prodeath or prosurvival. To understand the underlining mechanism of this dichotomy and its potential clinical implications, we treated various transformed and nontransformed human cells with proteasome inhibitors. We found that whether autophagy served a prosurvival role in this scenario was contingent on the cellular oncogenic status. Thus, autophagy suppression enhanced apoptosis induced by proteasome inhibitors in transformed cells, but not in nontransformed cells. Oncogenic transformation enhanced the ability of cells to initiate autophagy in response to stress, reflecting a stronger dependence of transformed cells on autophagy for survival. Indeed, a combined use of bortezomib, the only Food and Drug Administration-approved proteasome inhibitor for clinical use, and chloroquine, which inhibits autophagy by disturbing lysosomal functions, suppressed tumor growth more significantly than either agent alone in a xenograft model. These findings indicate that suppression of both intracellular degradation systems could constitute a novel strategy for enhanced cancer control in a tumor-specific way.
Figures
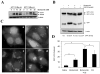
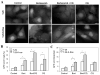
Figure 1. Induction of autophagy by Bortezomib
(A). Bax-positive and Bax–negative HCT116 cells were treated with different doses of Bortezomib for 16 hours. Total lysates were prepared and subjected to immunoblot analysis. (B). Bax-negative HCT116 stably expressing GFP-LC3 were treated with vehicle control or Bortezomib (20 nM) in the presence or absence of E64D (10 μM) and pepstatin A (10 μM) for 16 hours. Total cell lysates were subjected to immunoblot analysis using an anti-GFP antibody. Digital data (mean± SD) were from densitometry analysis of the GFP band from at least three independent experiments. (C-D). The same cells were treated with vehicle control (a), Bortezomib (20 nM, b), chloroquine (10 μM, d) or the combination of the two (c) for 16 hours and then examined by fluorescence microscopy (C). The numbers of GFP-LC3 dots per cell in each condition were quantified (D). Data (mean± SD) are representative of at least three independent experiments. *: p<0.01; #: p<0.05 (one way ANOVA with Scheffe's post-hoc test).
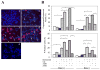
Figure 2. Bortezomib-induced autophagy is cytoprotective
(A). Bax-positive HCT116 cells were either untreated (a) or treated with Bortezomib (20 nM) alone (b) or in the presence of 3-MA (10 mM, c), CQ (10 μM, d), or z-VAD (50 μM, e) for 24 hours. Cell death was determined by nuclear staining with Hoechst 33342 for apoptotic cells with fragmented or condensed or nuclei (arrows), and by propidium iodide staining for cytoplasmic membrane permeability change. (B) Bax-positive and Bax-negative HCT116 cells were treated as in panel A and the percentages of cells with defined changes were quantified. Upper panel: apoptotic cells with fragmented or condensed or nuclei; lower panel; propidium iodide staining positive cells. (C). Bax-deficient HCT116 cells were transfected with a negative siRNA (Neg) or Beclin-1 (Bec) or LC3B-specific siRNA (120 nM) for 48 h and analyzed by immunoblot with indicated antibodies. (D). siRNA-transfected cells were then treated with Bortezomib (20 nM) for another 24 hours. Cell death was determined as in panel A. a: Neg siRNA only; b: Neg siRNA plus Bortezomib; c: siRNA against Beclin 1 plus Bortezomib and d: siRNA against LC3B plus Bortezomib. Arrows indicate fragmented or condensed nuclei. (E). siRNA-transfected cells were treated with Lactacystin (5 μM), ALLN (10 μM) or Bortezomib (20 nM) for another 24 hours. Cell death was determined as in panel A. Data (mean± SD) are representative of at least three independent experiments. *: p<0.01 (z test).
Figure 2. Bortezomib-induced autophagy is cytoprotective
(A). Bax-positive HCT116 cells were either untreated (a) or treated with Bortezomib (20 nM) alone (b) or in the presence of 3-MA (10 mM, c), CQ (10 μM, d), or z-VAD (50 μM, e) for 24 hours. Cell death was determined by nuclear staining with Hoechst 33342 for apoptotic cells with fragmented or condensed or nuclei (arrows), and by propidium iodide staining for cytoplasmic membrane permeability change. (B) Bax-positive and Bax-negative HCT116 cells were treated as in panel A and the percentages of cells with defined changes were quantified. Upper panel: apoptotic cells with fragmented or condensed or nuclei; lower panel; propidium iodide staining positive cells. (C). Bax-deficient HCT116 cells were transfected with a negative siRNA (Neg) or Beclin-1 (Bec) or LC3B-specific siRNA (120 nM) for 48 h and analyzed by immunoblot with indicated antibodies. (D). siRNA-transfected cells were then treated with Bortezomib (20 nM) for another 24 hours. Cell death was determined as in panel A. a: Neg siRNA only; b: Neg siRNA plus Bortezomib; c: siRNA against Beclin 1 plus Bortezomib and d: siRNA against LC3B plus Bortezomib. Arrows indicate fragmented or condensed nuclei. (E). siRNA-transfected cells were treated with Lactacystin (5 μM), ALLN (10 μM) or Bortezomib (20 nM) for another 24 hours. Cell death was determined as in panel A. Data (mean± SD) are representative of at least three independent experiments. *: p<0.01 (z test).
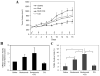
Figure 3. Combined suppression of the proteasome and autophagy enhances the inhibition of tumor growth in vivo
(A). Balb/c nude mice were implanted with 4 × 106 Bax-positive HCT116 cells on the right flank. Fourteen days after inoculations, mice were grouped and intraperitoneally given saline, Bortezomib (Bort, 0.33 m/kg), Bortezomib plus chloroquine (CQ, 45 mg/kg) or CQ alone every 3 days for 6 times as indicated by the arrows. The first treatment day is designated as day 0. Tumor volume (mm3) was determined every two days after the first treatment (day 0) until day 16 and the mean tumor volumes were calculated (n=5-6 per group). *: p<0.01 (Bortezomib/CQ group versus the saline group, one way ANOVA with Scheffe's post-hoc test). (B). Total lysates were prepared from each tumor sample and pooled in equal amount of protein within each group. The lysates were then analyzed for effector caspase activities using Ac-DEVD-AFC as the substrate. Data (mean± SD) of independent triplicate measurements were expressed as fold of increase over the saline control. (C). Tumor samples from each groups were subjected to TUNEL staining and the percentage of positive cells (mean± SD) were determined *: p<0.01 (z test). (D). Electron microscopic examination of tumor samples recovered from nude mice treated for 16 days with saline (a), Bortezomib (b), Bortezomib+CQ (c), or CQ only (d). Arrows: autophagic vesicles; N: nuclei. Scale bar: 1 μm. (E). The number of autophagic vesicles per 100 μm2 area was determined (means ± SD). *: p<0.01, #: p<0.02 (one way ANOVA with Scheffe's post-hoc test). (F). An equal amount of protein from each tumor sample within the same group (n=5-6) was combined and analyzed by immunoblot assay with anti-LC3 and anti-β-actin antibodies. Digital data (mean± SD) were from densitometry analysis of the LC3-II band from at least three independent experiments.
Figure 3. Combined suppression of the proteasome and autophagy enhances the inhibition of tumor growth in vivo
(A). Balb/c nude mice were implanted with 4 × 106 Bax-positive HCT116 cells on the right flank. Fourteen days after inoculations, mice were grouped and intraperitoneally given saline, Bortezomib (Bort, 0.33 m/kg), Bortezomib plus chloroquine (CQ, 45 mg/kg) or CQ alone every 3 days for 6 times as indicated by the arrows. The first treatment day is designated as day 0. Tumor volume (mm3) was determined every two days after the first treatment (day 0) until day 16 and the mean tumor volumes were calculated (n=5-6 per group). *: p<0.01 (Bortezomib/CQ group versus the saline group, one way ANOVA with Scheffe's post-hoc test). (B). Total lysates were prepared from each tumor sample and pooled in equal amount of protein within each group. The lysates were then analyzed for effector caspase activities using Ac-DEVD-AFC as the substrate. Data (mean± SD) of independent triplicate measurements were expressed as fold of increase over the saline control. (C). Tumor samples from each groups were subjected to TUNEL staining and the percentage of positive cells (mean± SD) were determined *: p<0.01 (z test). (D). Electron microscopic examination of tumor samples recovered from nude mice treated for 16 days with saline (a), Bortezomib (b), Bortezomib+CQ (c), or CQ only (d). Arrows: autophagic vesicles; N: nuclei. Scale bar: 1 μm. (E). The number of autophagic vesicles per 100 μm2 area was determined (means ± SD). *: p<0.01, #: p<0.02 (one way ANOVA with Scheffe's post-hoc test). (F). An equal amount of protein from each tumor sample within the same group (n=5-6) was combined and analyzed by immunoblot assay with anti-LC3 and anti-β-actin antibodies. Digital data (mean± SD) were from densitometry analysis of the LC3-II band from at least three independent experiments.
Figure 4. Suppression of autophagy in normal human peripheral blood mononuclear cells does not enhance the toxicity of proteasome inhibitors
(A). Human PBMC were incubated with MG132 (0.5 μM) or Bortezomib (20 nM) in the presence or absence of 3-MA (10 mM) for 24 hours. Immunoblot was then performed using the anti-LC3 antibody. (B-D). Human PBMC were treated with MG132 (0.5 μM), ALLN (10 μM) or Bortezomib (20 nM) in the presence or absence of 3-MA for 24 hours. Cell death was determined by propidium iodide staining (B) or by nuclear Hoechst 33342 staining for fragmented or condensed nuclei (C). Effector caspase activities were determined using Ac-DEVD-AFC as the substrate (D).
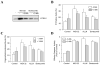
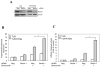
Figure 5. Transformed cells can initiate a more potent autophagic response than the matched non-transformed cells
(A). T-29 and T-29-K-Ras cells were first infected with Ad-GFP-LC3 for 24 hours and then treated with vehicle control, Bortezomib (20 nM), Bortezomib plus CQ (10 μM) or CQ alone for 24 hours. Cells were then analyzed by fluorescence microscopy for LC3 translocation. (B) The percentages (mean ± SD) of GFP-LC3-positive cells showing puncta were determined. *: p<0.01 (z test). (C) The numbers of GFP-LC3puncta per cells (mean ± SE) were determined. *: p<0.05 (Student's t test).
Figure 6. Suppression of autophagy promotes proteasome inhibitor-induced cell death in transformed but not in matched non-transformed cells
(A). T-29 and T-29-K-Ras cells were first transfected with a negative siRNA (Neg) or Beclin-1 specific siRNA (120 nM) for 48 hours. The total lysates were analyzed by immunoblot for the expression of Beclin 1 and β-actin. (B-C). siRNA-transfected cells were then treated with Bortezomib (40 nM) for another 24 hours. Cell death was then determined by nuclear Hoechst 33342 staining for fragmented or condensed nuclei (B). *: p<0.01 (z test). Effector caspase activities were measured using Ac-DEVD-AFC as the substrate (C). *: p<0.01 (one way ANOVA with Scheffe's post-hoc test).
Similar articles
- Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation.
Carew JS, Medina EC, Esquivel JA 2nd, Mahalingam D, Swords R, Kelly K, Zhang H, Huang P, Mita AC, Mita MM, Giles FJ, Nawrocki ST. Carew JS, et al. J Cell Mol Med. 2010 Oct;14(10):2448-59. doi: 10.1111/j.1582-4934.2009.00832.x. J Cell Mol Med. 2010. PMID: 19583815 Free PMC article. - Proteasome inhibitor interacts synergistically with autophagy inhibitor to suppress proliferation and induce apoptosis in hepatocellular carcinoma.
Hui B, Shi YH, Ding ZB, Zhou J, Gu CY, Peng YF, Yang H, Liu WR, Shi GM, Fan J. Hui B, et al. Cancer. 2012 Nov 15;118(22):5560-71. doi: 10.1002/cncr.27586. Epub 2012 Apr 19. Cancer. 2012. PMID: 22517429 - Inhibition of autophagy enhances apoptosis induced by proteasome inhibitor bortezomib in human glioblastoma U87 and U251 cells.
Zhang X, Li W, Wang C, Leng X, Lian S, Feng J, Li J, Wang H. Zhang X, et al. Mol Cell Biochem. 2014 Jan;385(1-2):265-75. doi: 10.1007/s11010-013-1835-z. Epub 2013 Oct 9. Mol Cell Biochem. 2014. PMID: 24104452 Free PMC article. - Novel proteasome inhibitors to overcome bortezomib resistance.
Ruschak AM, Slassi M, Kay LE, Schimmer AD. Ruschak AM, et al. J Natl Cancer Inst. 2011 Jul 6;103(13):1007-17. doi: 10.1093/jnci/djr160. Epub 2011 May 23. J Natl Cancer Inst. 2011. PMID: 21606441 Review. - Molecular crosstalk between the proteasome, aggresomes and autophagy: translational potential and clinical implications.
Driscoll JJ, Chowdhury RD. Driscoll JJ, et al. Cancer Lett. 2012 Dec 28;325(2):147-54. doi: 10.1016/j.canlet.2012.06.016. Epub 2012 Jul 7. Cancer Lett. 2012. PMID: 22781397 Review.
Cited by
- Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of head and neck cancer and their activities are enhanced by suppression of Mcl-1 or autophagy.
Zang Y, Thomas SM, Chan ET, Kirk CJ, Freilino ML, DeLancey HM, Grandis JR, Li C, Johnson DE. Zang Y, et al. Clin Cancer Res. 2012 Oct 15;18(20):5639-49. doi: 10.1158/1078-0432.CCR-12-1213. Epub 2012 Aug 28. Clin Cancer Res. 2012. PMID: 22929803 Free PMC article. - Autophagy as a target for anticancer therapy.
Janku F, McConkey DJ, Hong DS, Kurzrock R. Janku F, et al. Nat Rev Clin Oncol. 2011 May 17;8(9):528-39. doi: 10.1038/nrclinonc.2011.71. Nat Rev Clin Oncol. 2011. PMID: 21587219 Review. - Tumor cells can evade dependence on autophagy through adaptation.
Ding WX, Chen X, Yin XM. Ding WX, et al. Biochem Biophys Res Commun. 2012 Aug 31;425(3):684-8. doi: 10.1016/j.bbrc.2012.07.090. Epub 2012 Jul 25. Biochem Biophys Res Commun. 2012. PMID: 22842577 Free PMC article. - Dissecting the dynamic turnover of GFP-LC3 in the autolysosome.
Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX. Ni HM, et al. Autophagy. 2011 Feb;7(2):188-204. doi: 10.4161/auto.7.2.14181. Epub 2011 Feb 1. Autophagy. 2011. PMID: 21107021 Free PMC article. - Autophagy modulation for cancer therapy.
Yang ZJ, Chee CE, Huang S, Sinicrope F. Yang ZJ, et al. Cancer Biol Ther. 2011 Jan 15;11(2):169-76. doi: 10.4161/cbt.11.2.14663. Cancer Biol Ther. 2011. PMID: 21263212 Free PMC article. Review.
References
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. - PubMed
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–7. - PubMed
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA111456-04/CA/NCI NIH HHS/United States
- R01 CA083817-08/CA/NCI NIH HHS/United States
- CA111456/CA/NCI NIH HHS/United States
- R01 CA131183/CA/NCI NIH HHS/United States
- CA83817/CA/NCI NIH HHS/United States
- R01 CA111456/CA/NCI NIH HHS/United States
- R01 CA083817/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources