Matrix protein CCN1 is critical for prostate carcinoma cell proliferation and TRAIL-induced apoptosis - PubMed (original) (raw)
Matrix protein CCN1 is critical for prostate carcinoma cell proliferation and TRAIL-induced apoptosis
Carrie A Franzen et al. Mol Cancer Res. 2009 Jul.
Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) plays an important role in immune surveillance and preferentially induces apoptosis in cancer cells over normal cells, suggesting its potential in cancer therapy. However, the molecular basis for its selective killing of cancer cells is not well understood. Recent studies have identified the CCN family of integrin-binding matricellular proteins as important regulators of cell behavior, including cell adhesion, proliferation, migration, differentiation, and survival. We show here that CCN1 (CYR61) supports the adhesion of prostatic carcinoma cells as an adhesion substrate through integrins and heparan sulfate proteoglycans. Knockdown of CCN1 expression in PC-3 and DU-145 androgen-independent prostate cancer cells strongly inhibited their proliferation without causing apoptosis, indicating that CCN1 promotes their growth. However, CCN1 also significantly enhances TRAIL-induced apoptosis through interaction with integrins alphavbeta3 and alpha6beta4 and the cell-surface heparan sulfate proteoglycan syndecan-4, acting through a protein kinase Calpha-dependent mechanism without requiring de novo protein synthesis. Knockdown of CCN1 expression in PC-3, DU-145, and LNCaP cells severely blunted their sensitivity to TRAIL, an effect that was reversed by exogenously added CCN1 protein. These findings reveal a functional dichotomy for CCN1 in prostate carcinoma cells, because it contributes to both cell proliferation and TRAIL-induced cell death and suggest that CCN1 expression status may be an important parameter in assessing the efficacy of TRAIL-dependent cancer therapy.
Figures
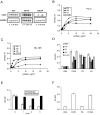
Figure 1. CCN1 is expressed in prostate cells and supports prostatic cell adhesion through integrins
A. RNA blot of total RNA from PC-3, DU145, and LNCaP cells treated with TPA (T; 10 nM), bFGF (bF; 10 ng/ml), EGF (E; 3 ng/ml), or TGF-β (Tb; 10 ng/ml) for 1 hr, hybridized to cDNA probes for CCN1 and GAPDH. B. PC-3 cells adhered on plates coated with various concentrations of CCN1, FN, VN, and LN for 30 min. Adherent cells were stained with methylene blue and extracted dye was quantified by absorbance at 620 nm. C. Adhesion of DU145 cells on various substrates as described above. D. PC-3 cells were pre-treated with antibodies against α6, β1, β4, or αvβ3, integrins before adhering to plates coated with indicated substrates and cell adhesion measured. E. PC3 cells were treated with 1-5 u/ml of heparinase or chondroitinase and their adhesion to CCN1-coated plates measured. F. PC-3 cell adhesion to plates coated with BSA, CCN1, or the CCN1 mutants TM (α6β1-HSPG binding-defective) and D125A (αv binding-defective) measured by methylene blue staining.
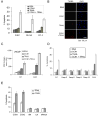
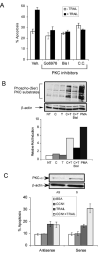
Figure 2. CCN1 is critical for proliferation of prostate carcinoma cells
PC-3 (A) and DU145 (D) cells were treated with anti-sense (AS) or sense (S) CCN1 oligonucleotides. To show CCN1 knockdown, cell lysates were collected 24 hrs after transfection and electrophorsed on 10% SDS-PAGE, followed by immunoblotting with antibodies against CCN1 and β-actin. Transfected PC-3 (B) and DU-145 (E) cells were cultured in growth media, and total cell numbers counted 24, 48, and 72 hrs later. Parallel plates of PC-3 (C) and DU-145 (F) cells were scored for apoptosis at each time point.
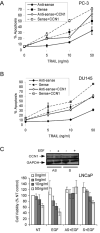
Figure 3. CCN1 cooperates with TRAIL to induce apoptosis in prostate cells
A. PrEC, DU145, and PC-3 cells were treated with CCN1 (10 μg/ml) and/or TRAIL (5 ng/ml) for 6 hrs and scored for apoptosis. B. PC-3 cells were treated with CCN1 and/or TRAIL as above, followed by TUNEL assay and counterstaining with DAPI. C. LNCaP cells were either untreated (NT) or treated with EGF for 60 min. prior to stimulation with CCN1 and/or TRAIL for 6 hrs, and subjected to M30 apoptosis assay. D. Cells were pre-treated with cycloheximide (5 μM) or caspase inhibitors for 30 min., including 50 μM Z-DEVD (caspase-3), Z-IETD (caspase-8), Z-LEHD (caspase-9), or Z-AEVD (caspase-10), then treated with CCN1 and/or TRAIL for 6 hrs as above and scored for apoptosis. E. PC-3 cells were adhered to plates coated with CCN1, CCN2, VN, LN (10 μg/ml each), or VN and LN and cultured in serum-free medium for 6 hrs with or without TRAIL (5 ng/ml). Cells were then fixed and scored for apoptosis.
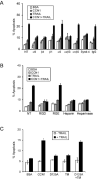
Figure 4. Downregulation of CCN1 in prostate carcinoma cells inhibits TRAIL-induced apoptosis
PC-3 (A) and DU145 (B) cells were transfected with sense or antisense CCN1 oligonucleotides as described in Figure 2, and treated with various concentrations of TRAIL (0-50 ng/ml) as indicted, either in the presence or absence of soluble CCN1 for 6 hrs prior to scoring for apoptosis by DAPI staining. C. LNCaP cells were transfected with sense or antisense CCN1 oligonucleotides and treated with EGF (3 ng/ml) where indicated for 1 hr before exposure to various concentrations of TRAIL and CCN1 (10 μg/ml) for 6 hrs. Cell viability was measured by the MTT assay. To show EGF induction of CCN1 and antisense knockdown, cell lysates were electrophorsed on 10% SDS-PAGE, followed by immunoblotting with antibodies against CCN1 and GAPDH (upper panel).
Figure 5. CCN1/TRAIL cooperation is mediated through αvβ3, α6β4, and syndecan-4
PC-3 cells were scored for apoptosis following indicated treatments. A. Cells were pre-treated with mAbs (50 μg/ml each) against α6 (GoH3), β4 (ASC-3), β1 (JB1A), α5β1 (JB-55), αvβ5 (P1F6), αvβ3 (anti-VNR-1), or syndecan-4 or mouse IgG for 1 hr prior to stimulation with CCN1 (10 μg/ml) and/or TRAIL (5 ng/ml). B. Cells were pre-treated for 30 min. with GRGDSP or GRGESP peptides (0.2 mM each), soluble heparin (1 mg/ml), or with heparinase (20 U/ml) for 24 hrs before addition of CCN1 and/or TRAIL. C. Cells were treated with WT CCN1, D125A (αv binding-defective mutant), TM (α6β1-HSPG binding-defective mutants), or D125A and TM with or without TRAIL and scored for apoptosis.
Figure 6. PKCα is required for CCN1/TRAIL cooperation
A. PC-3 cells were pre-treated with Gö6976 (1 μM), BisI (1 μM), or chelerythrine chloride (1 μM), or a vehicle control (Veh) and adhered to surfaces coated with CCN1 (10 μg/ml). TRAIL was then added and incubated for 6 hrs and scored for apoptosis. B. Adherent PC-3 cells were treated with CCN1 (C), TRAIL (T), CCN1 And TRAIL (C+T) or PMA for 30 min., with precincubation with BisI for 30 min where indicated. Cell lysates were resolved on 10% SDS-PAGE and immunoblotted with antibodies against phospho-(Ser)PKC substrates and β-actin. Graph shows quantification of signals using ImageJ software from NIH. C. PC-3 cells were transfected with anti-sense (AS) or sense (S) oligonucleotides against PKCα, treated with CCN1 and/or TRAIL for 6 hrs and scored for apoptosis. To analyze the oligonucleotide knockdown, cell lysates were collected 48 hrs after transfection and resolved on 10% SDS-PAGE, followed by immunoblotting with antibodies against PKCα or β-actin (upper panel).
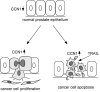
Figure 7. A model of CCN1 functions in prostate carcinoma cells
In the normal prostate epithelium, CCN1 expression is low. Elevated CCN1 expression (cells in grey) promotes prostate cancer cell proliferation, thus enhancing tumor growth. CCN1 is also a known angiogenic inducer and can upregulate the expression of MMPs (14;47), attributes that can promote tumor growth invasion. However, the expression of CCN1 makes the prostate cancer cells hypersensitive to TRAIL-induced apoptosis.
Similar articles
- CCN1/CYR61: the very model of a modern matricellular protein.
Lau LF. Lau LF. Cell Mol Life Sci. 2011 Oct;68(19):3149-63. doi: 10.1007/s00018-011-0778-3. Epub 2011 Jul 31. Cell Mol Life Sci. 2011. PMID: 21805345 Free PMC article. Review. - TNFα-induced apoptosis enabled by CCN1/CYR61: pathways of reactive oxygen species generation and cytochrome c release.
Juric V, Chen CC, Lau LF. Juric V, et al. PLoS One. 2012;7(2):e31303. doi: 10.1371/journal.pone.0031303. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363611 Free PMC article. - Fas-mediated apoptosis is regulated by the extracellular matrix protein CCN1 (CYR61) in vitro and in vivo.
Juric V, Chen CC, Lau LF. Juric V, et al. Mol Cell Biol. 2009 Jun;29(12):3266-79. doi: 10.1128/MCB.00064-09. Epub 2009 Apr 13. Mol Cell Biol. 2009. PMID: 19364818 Free PMC article. - CCN1 sensitizes esophageal cancer cells to TRAIL-mediated apoptosis.
Dang T, Modak C, Meng X, Wu J, Narvaez R, Chai J. Dang T, et al. Exp Cell Res. 2017 Dec 1;361(1):163-169. doi: 10.1016/j.yexcr.2017.10.015. Epub 2017 Oct 18. Exp Cell Res. 2017. PMID: 29055676 - Matricellular protein CCN1/CYR61: a new player in inflammation and leukocyte trafficking.
Emre Y, Imhof BA. Emre Y, et al. Semin Immunopathol. 2014 Mar;36(2):253-9. doi: 10.1007/s00281-014-0420-1. Epub 2014 Mar 18. Semin Immunopathol. 2014. PMID: 24638890 Review.
Cited by
- A sticky situation: CCN1 promotes both proliferation and apoptosis of cancer cells.
Leask A. Leask A. J Cell Commun Signal. 2010 Mar;4(1):71-2. doi: 10.1007/s12079-009-0079-x. Epub 2009 Oct 16. J Cell Commun Signal. 2010. PMID: 19834822 Free PMC article. - Postinfarction Hearts Are Protected by Premature Senescent Cardiomyocytes Via GATA 4-Dependent CCN 1 Secretion.
Cui S, Xue L, Yang F, Dai S, Han Z, Liu K, Liu B, Yuan Q, Cui Z, Zhang Y, Xu F, Chen Y. Cui S, et al. J Am Heart Assoc. 2018 Sep 18;7(18):e009111. doi: 10.1161/JAHA.118.009111. J Am Heart Assoc. 2018. PMID: 30371213 Free PMC article. - CCN1/CYR61: the very model of a modern matricellular protein.
Lau LF. Lau LF. Cell Mol Life Sci. 2011 Oct;68(19):3149-63. doi: 10.1007/s00018-011-0778-3. Epub 2011 Jul 31. Cell Mol Life Sci. 2011. PMID: 21805345 Free PMC article. Review. - The matricellular protein CCN6 (WISP3) decreases Notch1 and suppresses breast cancer initiating cells.
Huang W, Martin EE, Burman B, Gonzalez ME, Kleer CG. Huang W, et al. Oncotarget. 2016 May 3;7(18):25180-93. doi: 10.18632/oncotarget.7734. Oncotarget. 2016. PMID: 26933820 Free PMC article. - A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis.
Taroni JN, Greene CS, Martyanov V, Wood TA, Christmann RB, Farber HW, Lafyatis RA, Denton CP, Hinchcliff ME, Pioli PA, Mahoney JM, Whitfield ML. Taroni JN, et al. Genome Med. 2017 Mar 23;9(1):27. doi: 10.1186/s13073-017-0417-1. Genome Med. 2017. PMID: 28330499 Free PMC article.
References
- Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006 Mar 15;12(6):1665–71. - PubMed
- Damber JE, Aus G. Prostate cancer. Lancet. 2008 May 17;371(9625):1710–21. - PubMed
- Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39(78):1462–75. - PubMed
- Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002 Feb 1;168(3):1356–61. - PubMed
- Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature's TRAIL--on a path to cancer immunotherapy. Immunity. 2003 Jan;18(1):1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM078492/GM/NIGMS NIH HHS/United States
- R01 CA046565-19/CA/NCI NIH HHS/United States
- R01 GM078492-02/GM/NIGMS NIH HHS/United States
- R01 CA046565/CA/NCI NIH HHS/United States
- GM78492/GM/NIGMS NIH HHS/United States
- CA46565/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical