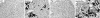Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model - PubMed (original) (raw)
Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model
Teruyo Nakatani et al. FASEB J. 2009 Nov.
Abstract
Hyp mice possess a mutation that inactivates the phosphate-regulating gene, which is homologous to the endopeptidases of the X-chromosome (PHEX). The mutation is associated with severe hypophosphatemia due to excessive urinary phosphate wasting. Such urinary phosphate wasting in Hyp mice is associated with an increased serum accumulation of fibroblast growth factor (FGF) 23. We wanted to determine the biological significance of increased serum FGF23 levels and concomitant hypophosphatemia in Hyp mice and to evaluate whether FGF23 activity could be modified by manipulating klotho (a cofactor of FGF23 signaling). We generated Hyp and klotho double-mutant mice (Hyp/klotho(-/-)). Severe hypophosphatemia of Hyp mice was reversed to hyperphosphatemia in Hyp/klotho(-/-) double mutants, despite the fact that the double mutants showed significantly increased serum levels of FGF23. Hyperphosphatemia in Hyp/klotho(-/-) mice was associated with increased renal expression of sodium/phosphate cotransporter 2a (NaPi2a) protein. Exogenous injection of bioactive parathyroid hormone 1-34 down-regulated renal expression of NaPi2a and consequently reduced serum levels of phosphate in Hyp/klotho(-/-) mice. Moreover, in contrast to the Hyp mice, the Hyp/klotho(-/-) mice showed significantly higher serum levels of 1,25-dihydroxyvitamin D and developed extensive calcification in soft tissues and vascular walls. Furthermore, compared with the Hyp mice, Hyp/klotho(-/-) mice were smaller in size, showed features of generalized tissue atrophy, and generally died by 15-20 wk of age. Our in vivo studies provide genetic evidence for a pathological role of increased FGF23 activities in regulating abnormal phosphate homeostasis in Hyp mice. Moreover, these results suggest that even when serum levels of FGF23 are significantly high, in the absence of klotho, FGF23 is unable to regulate systemic phosphate homeostasis. Our in vivo observations have significant clinical implications in diseases associated with increased FGF23 activity and suggest that the functions of FGF23 can be therapeutically modulated by manipulating the effects of klotho.
Figures
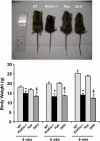
Figure 1.
Macroscopic phenotype of Hyp/_klotho_−/− double mutants. Top panel: gross phenotype of wild-type (WT), _klotho_−/−, Hyp, and _Hyp/klotho_−/− (DKO) mice at 6 wk of age. Bottom panel: body weights of 4-, 6-, and 9-wk-old WT, _klotho_−/−, and _Hyp/klotho_−/− and Hyp mice, showing that _Hyp/klotho_−/− mice are smaller than Hyp mice. These observations suggest that inactivation of klotho function in Hyp mice can induce body weight loss in Hyp/klotho_−/− mice. *P < 0.01 vs. WT mice; §_P < 0.01 vs. Hyp mice.
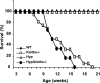
Figure 2.
Survival of various genotypes. Survival for wild-type (WT) (_n_=11), _klotho_−/− (_n_=13), and Hyp/_klotho_−/− (DKO, _n_=7) and Hyp (_n_=18) mice. Survival of Hyp/_klotho_−/− double-knockout mice is far lower than that of WT and Hyp mice and is similar to that of _klotho_−/− mice. Most of the Hyp/_klotho_−/−and _klotho_−/−mice died by 15 to 20 wk of age, whereas none of the WT and Hyp mice had died by the end of the 25-wk observation period. Mice used to generate the survival curve were not always littermates but were of similar genetic background.
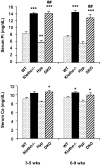
Figure 3.
Biochemical measurements of serum phosphate and calcium levels in Hyp/_klotho_−/− mice. Note that serum phosphate (top panel) and calcium (bottom panel) levels are higher in _klotho_−/− mice than in the wild-type (WT) mice. Serum phosphate level is significantly higher in _klotho_−/− mice (14.±0.2 mg/dl) than in WT mice (8.3±0.4 mg/dl), at 3–5 wk of age. Similar hyperphosphatemia is also observed in _klotho_−/− mice (14.4±0.5 mg/dl) at 6–9 wk of age, compared with WT mice (7.2±0.4 mg/dl) of the same age. In contrast to the level in Hyp mice at 3–5 wk (5.7±0.57 mg/dl) and 6–9 wk (5.4±0.4 mg/dl), serum phosphate level is significantly increased in _Hyp/klotho_−/− (DKO) mice, both at 3–5 wk (14±0.6 mg/dl) and 6–9 wk (12.8±0.8 mg/dl) of age. Serum calcium level is higher in _klotho_−/− mice (10.4±0.3 mg/dl) at 6–9 wk of age than in WT mice (9.4±0.1 mg/dl) (bottom panel). Similar elevated serum calcium levels are also observed in _Hyp/klotho_−/− double-mutant mice (9.9±0.4 mg/dl) at 6–9 wk of age. In contrast to _Hyp/klotho_−/− double-mutant mice at 6–9 wk, serum calcium level is lower in Hyp mice at 6–9 wk (8.4±0.3 mg/dl) of age. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT; ##P < 0.001 vs. Hyp.
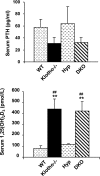
Figure 4.
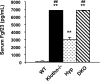
Biochemical measurements of serum PTH and 1,25(OH)2D3 from various genotypes. Compared with those in wild-type (WT) mice (_n_=24; 57.8±13 pg/ml), serum PTH levels are reduced in _klotho_−/− mice (_n_=22; 31.3±9 pg/ml). Serum PTH levels are similar in WT and Hyp mice (_n_=11; 64.2±28 pg/ml). In contrast, serum PTH levels are reduced in _Hyp/klotho_−/− (DKO) mice (_n_=10; 32.5±8 pg/ml) compared with those in Hyp mice. Compared with levels in WT mice (_n_=5; 80.7±20 pM) and Hyp mice (_n_=5; 116.0±9.4 pM), serum 1,25(OH)2D3 levels were markedly increased in both _klotho_−/− mice (_n_=5; 441.8±89 pM) and _Hyp/klotho_−/− mice (_n_=5; 424.6±80 pM). **P < 0.001 vs. WT; ##P < 0.01 vs. Hyp.
Figure 5.
Biochemical measurements of serum FGF23 in various genotypes. Average serum levels of FGF23 (after adjusting dilution factors) are higher in _klotho_−/− mice (_n_=5; ∼6500 pg/ml) than in wild-type (WT) mice (_n_=4; ∼150 pg/ml). Similarly increased FGF23 serum levels are also noted in Hyp mice (_n_=5; ∼3000 pg/ml) and _Hyp/klotho_−/− (DKO) mice (_n_=5; ∼6500 pg/ml). **P < 0.001 vs. WT; ##P < 0.001 vs. Hyp.
Figure 6.
Expression of NaPi2a. Immunostaining for NaPi2a in the kidney sections prepared from the wild-type (WT), _klotho_−/−, Hyp, and _Hyp/klotho_−/− (DKO) mice. Expression of NaPi2a protein is significantly increased in the luminal side of the proximal tubular epithelial cells of _klotho_−/− and _Hyp/klotho_−/− mice, in contrast with the reduced expression seen in Hyp mice. Note that PTH injection suppressed NaPi2a expression in both _klotho_−/− and _Hyp/klotho_−/− mice. Kidney sections prepared from _NaPi2a_−/− mice did not show any specific staining.
Figure 7.
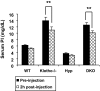
Bioactive PTH injection: serum phosphate levels before and 2 h after injection with bioactive PTH(1-34) protein. PTH protein injection resulted in significantly lowered serum phosphate levels in _klotho_−/− mice (13.8±1.0 vs. 10.9±0.94 mg/dl; P<0.01) and _Hyp/klotho_−/− (DKO) mice (12.5±0.7 vs. 10.1±0.6 mg/dl; P<0.01), suggesting that PTH controls systemic phosphate homeostasis by influencing NaPi2a activities. **P < 0.01 vs. corresponding preinjection value.
Figure 8.
Von Kossa staining of kidney tissues. Renal sections prepared from wild-type (WT), _klotho_−/−, Hyp, and _Hyp/klotho_−/− mice, with extensive calcification seen in kidneys of _klotho_−/− and _Hyp/klotho_−/− mice. No such renal calcification is found in Hyp or WT mice (×20).
Figure 9.
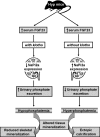
Regulation of phosphate homeostasis in Hyp mice. Schematic diagram outlining the possible molecular and biochemical events that may be involved in inducing different phenotypes in the Hyp mice in the presence or absence of klotho.
Similar articles
- In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels.
Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Ohnishi M, et al. Circ Cardiovasc Genet. 2009 Dec;2(6):583-90. doi: 10.1161/CIRCGENETICS.108.847814. Epub 2009 Sep 21. Circ Cardiovasc Genet. 2009. PMID: 20031638 Free PMC article. - FGF23-induced hypophosphatemia persists in Hyp mice deficient in the WNT coreceptor Lrp6.
Uchihashi K, Nakatani T, Goetz R, Mohammadi M, He X, Razzaque MS. Uchihashi K, et al. Contrib Nephrol. 2013;180:124-37. doi: 10.1159/000346792. Epub 2013 May 3. Contrib Nephrol. 2013. PMID: 23652555 Free PMC article. - 1,25-Dihydroxyvitamin D Maintains Brush Border Membrane NaPi2a and Attenuates Phosphaturia in Hyp Mice.
Martins JS, Liu ES, Sneddon WB, Friedman PA, Demay MB. Martins JS, et al. Endocrinology. 2019 Oct 1;160(10):2204-2214. doi: 10.1210/en.2019-00186. Endocrinology. 2019. PMID: 31237611 Free PMC article. - Does Fgf23-klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism?
Memon F, El-Abbadi M, Nakatani T, Taguchi T, Lanske B, Razzaque MS. Memon F, et al. Kidney Int. 2008 Sep;74(5):566-70. doi: 10.1038/ki.2008.218. Epub 2008 Jun 4. Kidney Int. 2008. PMID: 18528324 Free PMC article. Review. - Osteo-renal cross-talk and phosphate metabolism by the FGF23-Klotho system.
Ohnishi M, Razzaque MS. Ohnishi M, et al. Contrib Nephrol. 2013;180:1-13. doi: 10.1159/000346774. Epub 2013 May 3. Contrib Nephrol. 2013. PMID: 23652546 Review.
Cited by
- Vascular Calcification-New Insights Into Its Mechanism.
Lee SJ, Lee IK, Jeon JH. Lee SJ, et al. Int J Mol Sci. 2020 Apr 13;21(8):2685. doi: 10.3390/ijms21082685. Int J Mol Sci. 2020. PMID: 32294899 Free PMC article. Review. - Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging.
Ohnishi M, Razzaque MS. Ohnishi M, et al. FASEB J. 2010 Sep;24(9):3562-71. doi: 10.1096/fj.09-152488. Epub 2010 Apr 23. FASEB J. 2010. PMID: 20418498 Free PMC article. - Therapeutic potential of klotho-FGF23 fusion polypeptides: WO2009095372.
Razzaque MS. Razzaque MS. Expert Opin Ther Pat. 2010 Jul;20(7):981-5. doi: 10.1517/13543771003774100. Expert Opin Ther Pat. 2010. PMID: 20459364 Free PMC article. - FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH.
Yuan Q, Sato T, Densmore M, Saito H, Schüler C, Erben RG, Lanske B. Yuan Q, et al. J Bone Miner Res. 2011 Sep;26(9):2026-35. doi: 10.1002/jbmr.433. J Bone Miner Res. 2011. PMID: 21590742 Free PMC article. - Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity.
Brown RB, Razzaque MS. Brown RB, et al. Bonekey Rep. 2015 Jun 3;4:705. doi: 10.1038/bonekey.2015.74. eCollection 2015. Bonekey Rep. 2015. PMID: 26131357 Free PMC article. Review.
References
- Yu X, White K E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 2005;16:221–232. - PubMed
- Fukumoto S. Physiological regulation and disorders of phosphate metabolism—pivotal role of fibroblast growth factor 23. Intern Med. 2008;47:337–343. - PubMed
- Razzaque M S, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. - PubMed
- Gaasbeek A, Meinders A E. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–1101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases