Build-ups in the supply chain of the brain: on the neuroenergetic cause of obesity and type 2 diabetes mellitus - PubMed (original) (raw)
Build-ups in the supply chain of the brain: on the neuroenergetic cause of obesity and type 2 diabetes mellitus
Achim Peters et al. Front Neuroenergetics. 2009.
Abstract
Obesity and type 2 diabetes have become the major health problems in many industrialized countries. A few theoretical frameworks have been set up to derive the possible determinative cause of obesity. One concept views that food availability determines food intake, i.e. that obesity is the result of an external energy "push" into the body. Another one views that the energy milieu within the human organism determines food intake, i.e. that obesity is due to an excessive "pull" from inside the organism. Here we present the unconventional concept that a healthy organism is maintained by a "competent brain-pull" which serves systemic homeostasis, and that the underlying cause of obesity is "incompetent brain-pull", i.e. that the brain is unable to properly demand glucose from the body. We describe the energy fluxes from the environment, through the body, towards the brain with a mathematical "supply chain" model and test whether its predictions fit medical and experimental data sets from our and other research groups. In this way, we show data-based support of our hypothesis, which states that under conditions of food abundance incompetent brain-pull will lead to build-ups in the supply chain culminating in obesity and type 2 diabetes. In the same way, we demonstrate support of the related hypothesis, which states that under conditions of food deprivation a competent brain-pull mechanism is indispensable for the continuance of the brain s high energy level. In conclusion, we took the viewpoint of integrative physiology and provided evidence for the necessity of brain-pull mechanisms for the benefit of health. Along these lines, our work supports recent molecular findings from the field of neuroenergetics and continues the work on the "Selfish Brain" theory dealing with the maintenance of the cerebral and peripheral energy homeostasis.
Keywords: Selfish Brain theory; brain metabolism; brain-pull; diabetes mellitus; experimental human study; glucose allocation; obesity; supply chain.
Figures
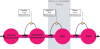
Figure 1
General supply chain of the human brain. Energy from the remote environment is brought to the immediate environment, it is then taken up by the body, and from there a large part of it enters the brain. In a general supply chain, the flux of energy can principally be determined by the supplier (previous step) or by the receiver (proximate step). The share of the flux which is determined by the supplier is called the “push component” (blue arrows), the share which is determined by the receiver is called the “pull component” (black arrows). The glucostatic and lipostatic theory cover a particular section (gray area) within the extended supply chain of the brain. A principle is adherent to general supply chains: The flow of goods (energy) is directed antegrade (towards the final consumer), but in case of an interruption at some point, the disturbance propagates retrograde, in the opposite direction, i.e. away from the consumer. In this way, build-ups can develop in front of the “bottleneck.”
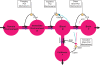
Figure 2
Flow-chart of the brain-supply-chain model. The graphical representation of the mathematical supply-chain model displays the branching of the energy flow between the brain and the side buffer, i.e. the fat/muscle compartment. In the “Discussion” section we will refer to experiments identifying distinct biological mechanisms that can fulfil specific pull-functions in the model. In short, the mechanisms are organized to satisfy the needs of the respective compartment: Firstly, upon the fall of astrocytic ATP concentrations, e.g. due to neuronal excitation, astrocytes use direct brain-pull mechanisms. Secondly, upon a fall of ATP concentrations in the brain, the allocative brain-pull mechanisms are activated (via red dotted arrow). The core of these mechanisms is represented by the ventromedial hypothalamus (VMH) and the sympatho-adrenal system (SNS), which inhibits peripheral glucose uptake in order to enhanced the supply of the brain. Thirdly, upon a fall of the blood glucose concentration, ingestive pull mechanisms in the lateral hypothalamus (LH) are initiated, i.e. food is taken up. Fourthly, upon energy deprivation in the peripheral buffers, the fall of leptin also promotes ingestive behaviour (via green dotted arrow) and prepares peripheral glucose uptake. When blood glucose concentrations rise after food intake, insulin functions as an energy-storage hormone and mediates a push-mechanism, which drives glucose in a glucose-dependent manner from blood to fat/muscle. Fifthly, upon recognition of food deprivation in the immediate vicinity, foraging pull mechanisms are initiated for locomotion and orientation.
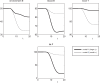
Figure 3
The effect of brain-pull efficiency on the maintenance of cerebral homeostasis under the condition of food restriction in a “supply chain” simulation study. The model is non-dimensional; time is denoted as t. In model 1 (bold line), there is an efficient brain-pull component, in model 2 (thin line) the brain-pull component is inefficient. For both models, the nutritional availability in the environment is set to be restricted from a certain time point onwards. In model 1, the supply-chain model predicts a fall of the blood glucose concentration and a decrease of the energy content in the fat/muscle compartment. The energy content in the brain is only marginally reduced, i.e. energetic brain homeostasis is maintained. In model 2, however, the model predicts a considerable disturbance of the brain energy homeostasis. Besides, the energy uptake from the environment is smaller in model 1 than in model 2, indicating a more economic utilisation of the food offer.
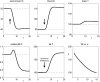
Figure 4
The effect of brain-pull inefficiency on the development of obesity and diabetes under the condition of food abundance in a “supply chain” simulation study. In both cases, the food offer in the environment is set to be abundant. In case 1 (dashed line; large α) there is an efficient allocative brain-pull mechanism present, in case 2 (bold line; small α) this brain-pull mechanism is inefficient from a certain moment onwards (indicated by the arrow). In case 1, with normal allocative brain-pull, the model predicts stably normal energy content in blood, muscle, fat, and brain. In case 2, with a disrupted ATP-dependency of the allocative brain-pull mechanisms, the model predicts accumulation of energy in the fat and muscle stores and also an accumulation of energy in the blood vessels, i.e. hyperglycaemia. Despite all these changes in case 2, the model predicts the maintenance of cerebral homeostasis. Bottom left: Peripheral glucose uptake jBF/F decreases in the long-term – a phenomenon commonly referred to as “insulin resistance”. Bottom right: Increasing fat compartment with decreasing brain-pull efficiency (α).
Similar articles
- The selfish brain: Competition for energy resources.
Peters A. Peters A. Am J Hum Biol. 2011 Jan-Feb;23(1):29-34. doi: 10.1002/ajhb.21106. Am J Hum Biol. 2011. PMID: 21080380 Review. - The selfish brain: stress and eating behavior.
Peters A, Kubera B, Hubold C, Langemann D. Peters A, et al. Front Neurosci. 2011 May 30;5:74. doi: 10.3389/fnins.2011.00074. eCollection 2011. Front Neurosci. 2011. PMID: 21660101 Free PMC article. - The selfish brain: competition for energy resources.
Fehm HL, Kern W, Peters A. Fehm HL, et al. Prog Brain Res. 2006;153:129-40. doi: 10.1016/S0079-6123(06)53007-9. Prog Brain Res. 2006. PMID: 16876572 Review. - Low plasma lactate concentration as a biomarker of an incompetent brain-pull: a risk factor for weight gain in type 2 diabetes patients.
van Dyken R, Hubold C, Meier S, Hitze B, Marxsen A, Oltmanns KM, Schweiger U, Lehnert H, Pellerin L, Peters A. van Dyken R, et al. Psychoneuroendocrinology. 2010 Oct;35(9):1287-93. doi: 10.1016/j.psyneuen.2010.02.016. Epub 2010 Mar 17. Psychoneuroendocrinology. 2010. PMID: 20299158 Clinical Trial. - The selfish brain: competition for energy resources.
Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. Peters A, et al. Neurosci Biobehav Rev. 2004 Apr;28(2):143-80. doi: 10.1016/j.neubiorev.2004.03.002. Neurosci Biobehav Rev. 2004. PMID: 15172762 Review.
Cited by
- The corpulent phenotype-how the brain maximizes survival in stressful environments.
Peters A, Kubera B, Hubold C, Langemann D. Peters A, et al. Front Neurosci. 2013 Apr 2;7:47. doi: 10.3389/fnins.2013.00047. eCollection 2013. Front Neurosci. 2013. PMID: 23565074 Free PMC article. - Systemic investigation of a brain-centered model of the human energy metabolism.
Göbel B, Langemann D. Göbel B, et al. Theory Biosci. 2011 Mar;130(1):5-18. doi: 10.1007/s12064-010-0105-9. Epub 2010 Aug 24. Theory Biosci. 2011. PMID: 20734159 Review. - How Stress Can Change Our Deepest Preferences: Stress Habituation Explained Using the Free Energy Principle.
Hartwig M, Bhat A, Peters A. Hartwig M, et al. Front Psychol. 2022 May 31;13:865203. doi: 10.3389/fpsyg.2022.865203. eCollection 2022. Front Psychol. 2022. PMID: 35712161 Free PMC article. Review. - Insulin resistance, selfish brain, and selfish immune system: an evolutionarily positively selected program used in chronic inflammatory diseases.
Straub RH. Straub RH. Arthritis Res Ther. 2014 Nov 13;16 Suppl 2(Suppl 2):S4. doi: 10.1186/ar4688. Arthritis Res Ther. 2014. PMID: 25608958 Free PMC article. Review. - Proximal Disruption of Brain Energy Supply Raises Systemic Blood Glucose: A Systematic Review.
Sprengell M, Kubera B, Peters A. Sprengell M, et al. Front Neurosci. 2021 Jun 24;15:685031. doi: 10.3389/fnins.2021.685031. eCollection 2021. Front Neurosci. 2021. PMID: 34248487 Free PMC article.
References
- Aiello L. C., Wheeler P. (1995). The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199–22110.1086/204350 - DOI
LinkOut - more resources
Full Text Sources