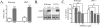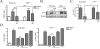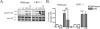Microglial low-density lipoprotein receptor-related protein 1 modulates c-Jun N-terminal kinase activation - PubMed (original) (raw)
Microglial low-density lipoprotein receptor-related protein 1 modulates c-Jun N-terminal kinase activation
Ana Pocivavsek et al. J Neuroimmunol. 2009.
Abstract
Apolipoprotein E (apoE)-induced activation of low-density lipoprotein receptor (LDL) family members reduces inflammatory responses by suppressing c-Jun N-terminal kinase (JNK) activation. We aimed to identify which specific receptor family member mediates the effect of apoE on inflammation in primary cultures of microglia. Low-density lipoprotein receptor-related protein 1 (LRP1)-deficient (LRP1-/-) microglia were derived from mice using tissue-specific loxP/Cre recombination. Using a peptide formed from the receptor-binding region of apoE (EP), we found that LRP1 mediates the effects of apoE on microglial inflammation. Microglial LRP1 was also essential for EP to suppress JNK activation induced by lipopolysaccharide.
Figures
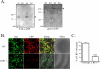
Figure 1. LRP1 knockout in primary microglia
A) Cell lysates from wild-type (wt) and LRP1 −/− (ko) microglia were analyzed by Western blotting for LRP1 expression (denoted by the asterisks). The left panel shows a representative blot probed with 5A6, recognizing the 85 kDa band for LRP1. The right panel shows a blot probed with goat α-LRP1, recognizing full length LRP1 (n = 4). B) Primary microglia from wild-type (WT) and LRP1 −/− cultures were immunostained with monoclonal Iba1 (detected with Alexa488, green) and goat anti-LRP antibody (detected with Alexa568, red). C) Cells positive for Iba1 and LRP1 expression were counted. Quantification shows that 95 % of wild-type microglia and 25 % of LRP1 −/− microglia were LRP1 positive (mean ± SEM; *** P < 0.001 compared to wild-type; n = 5 for wild-type and n = 7 for LRP1 −/−).
Figure 2. Stimulation of microglia with LPS increased NO through JNK activation
A) In wild-type and LRP1 −/− microglia, LPS promoted nitrite accumulation in the conditioned media at 24 h (mean ± SEM; ***P < 0.001; n = 6 for each of 3 independent experiments). B) Cell lysates from wild-type and LRP1 −/− microglia were analyzed by Western blotting with antibodies to iNOS and β-actin (as a control). A representative blot shows that LPS increased iNOS levels in both cell types (n=6 for each of 3 independent experiments). C) Wild-type and LRP1 −/− microglia were treated with LPS alone (open bars) or LPS and SP600125 (colored bars). In a dose-dependent manner, SP600125 treatment reduced nitrite production in both wild-type and LRP1 −/− microglia to levels of unstimulated microglia (defined as the line at 100 %) (mean ± SEM; **P < 0.01 compared to control cultures; # P < 0.05, ## P < 0.01 compared to corresponding cultures; n=3 for each of 2 independent experiments).
Figure 3. ApoE peptide attenuated LPS-induced NO production in LRP1 expressing microglia
A) Wild-type and LRP1 −/− microglia were treated with 100 ng/mL LPS and nitrite was measured in the conditioned media at 24 h. LPS induced nitrite accumulation in both cell types and EP showed attenuation of LPS-induced nitrite production only in wild-type cultures (mean ± SEM; *** P < 0.001 compared to corresponding control cultures; ## P < 0.01, ### P < 0.001 compared with indicated cultures; n = 6 for each of 4 independent experiments). B) Cell lysates from wild-type and LRP1 −/− cultures were analyzed by Western blotting for iNOS and b-actin. Representative blots show that EP and LPS treatment reduced iNOS expression compared to LPS stimulation alone in wild-type stimulated cultures but not LRP1 −/− cultures. C) Western blot data were quantified as percent of control (mean ± SEM; * P < 0.05, ** P < 0.01 compared with LPS stimulated cultures; # < 0.05 compared with indicated cultures; n = 6 for each of 4 independent experiments). D) Wild-type (left panel) and LRP1 −/− (right panel) cultures were treated LPS and nitrite was measured in the conditioned media at 24 h. LPS induced nitrite accumulation in wild-type and LRP1 −/− cultures. EP and LPS treatment attenuated LPS-induced nitrite accumulation significantly in wild-type cultures and treatment with 500 nM VLDLR blocking antibody, EP and LPS did not prevent the effects of EP. Similarly in LRP1 −/− microglia, nitrite accumulation in cultures treated with VLDLR antibody cocktail did not significantly differ from cultures treated with EP and LPS (mean ± SEM; ns means not significant; n = 4).
Figure 4. ApoE peptide signaling effects are LRP1 mediated
Wild-type and LRP1 −/− primary microglia were treated with PBS (c), 1 μM EP, 100 ng/mL LPS, and 1 μM EP with LPS for 1 h. Cell lysates were analyzed by Western blotting with antibodies to phospho-JNK and total JNK. A) Wild-type cells treated with EP showed decreased phospho-JNK compared with cells treated with PBS (c). Treatment of wild-type cells with LPS increased phospho-JNK. Cells treated with EP and LPS showed reduced phospho-JNK compared to cells treated with LPS alone. LRP1 −/− cells treated with LPS showed increased phospho-JNK compared with cells treated with PBS (c). In LRP1 −/− cultures, EP and LPS treatment did not reduce phospho-JNK compared to cells treated with LPS alone. B) Western blot data were quantified as percent of control phospho-JNK (mean ± SEM; *** P < 0.001 compared with control cultures; ## P < 0.01 compared with indicated cultures; n = 3 for each of 2 independent experiments).
Similar articles
- Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase.
Pocivavsek A, Burns MP, Rebeck GW. Pocivavsek A, et al. Glia. 2009 Mar;57(4):444-53. doi: 10.1002/glia.20772. Glia. 2009. PMID: 18803301 Free PMC article. - LRP1 modulates the microglial immune response via regulation of JNK and NF-κB signaling pathways.
Yang L, Liu CC, Zheng H, Kanekiyo T, Atagi Y, Jia L, Wang D, N'songo A, Can D, Xu H, Chen XF, Bu G. Yang L, et al. J Neuroinflammation. 2016 Dec 8;13(1):304. doi: 10.1186/s12974-016-0772-7. J Neuroinflammation. 2016. PMID: 27931217 Free PMC article. - The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain.
Zhang C, An J, Strickland DK, Yepes M. Zhang C, et al. Am J Pathol. 2009 Feb;174(2):586-94. doi: 10.2353/ajpath.2009.080661. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147818 Free PMC article. - 2'-Hydroxycinnamaldehyde targets low-density lipoprotein receptor-related protein-1 to inhibit lipopolysaccharide-induced microglial activation.
Hwang H, Jeon H, Ock J, Hong SH, Han YM, Kwon BM, Lee WH, Lee MS, Suk K. Hwang H, et al. J Neuroimmunol. 2011 Jan;230(1-2):52-64. doi: 10.1016/j.jneuroim.2010.08.021. Epub 2010 Oct 8. J Neuroimmunol. 2011. PMID: 20933287 - LDL receptor-related protein-1: a regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system.
Gonias SL, Campana WM. Gonias SL, et al. Am J Pathol. 2014 Jan;184(1):18-27. doi: 10.1016/j.ajpath.2013.08.029. Epub 2013 Oct 12. Am J Pathol. 2014. PMID: 24128688 Free PMC article. Review.
Cited by
- A multi-hit hypothesis for an APOE4-dependent pathophysiological state.
Steele OG, Stuart AC, Minkley L, Shaw K, Bonnar O, Anderle S, Penn AC, Rusted J, Serpell L, Hall C, King S. Steele OG, et al. Eur J Neurosci. 2022 Nov;56(9):5476-5515. doi: 10.1111/ejn.15685. Epub 2022 Jun 6. Eur J Neurosci. 2022. PMID: 35510513 Free PMC article. Review. - LRP-1 promotes cancer cell invasion by supporting ERK and inhibiting JNK signaling pathways.
Langlois B, Perrot G, Schneider C, Henriet P, Emonard H, Martiny L, Dedieu S. Langlois B, et al. PLoS One. 2010 Jul 14;5(7):e11584. doi: 10.1371/journal.pone.0011584. PLoS One. 2010. PMID: 20644732 Free PMC article. - APOE Genotype Alters Immunoglobulin Subtypes in Knock-In Mice.
Zhou Y, Zhao W, Al-Muhtasib N, Rebeck GW. Zhou Y, et al. J Alzheimers Dis. 2015;46(2):365-74. doi: 10.3233/JAD-142184. J Alzheimers Dis. 2015. PMID: 25737044 Free PMC article. - Low Density Lipoprotein-Receptor Related Protein 1 Is Differentially Expressed by Neuronal and Glial Populations in the Developing and Mature Mouse Central Nervous System.
Auderset L, Cullen CL, Young KM. Auderset L, et al. PLoS One. 2016 Jun 9;11(6):e0155878. doi: 10.1371/journal.pone.0155878. eCollection 2016. PLoS One. 2016. PMID: 27280679 Free PMC article. - APOE genotype alters glial activation and loss of synaptic markers in mice.
Zhu Y, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, LaDu MJ. Zhu Y, et al. Glia. 2012 Apr;60(4):559-69. doi: 10.1002/glia.22289. Epub 2012 Jan 6. Glia. 2012. PMID: 22228589 Free PMC article.
References
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimer's amyloid beta-protein by microglia in culture. J Neurosci Res. 1996;43:190–202. - PubMed
- Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. LRP and senile plaques in Alzheimer's disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104:38–46. - PubMed
- Bales KR, Du Y, Holtzman D, Cordell B, Paul SM. Neuroinflammation and Alzheimer's disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiol Aging. 2000;21:427–432. discussion 451−423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL050784/HL/NHLBI NIH HHS/United States
- AG14473/AG/NIA NIH HHS/United States
- R01 HL050784/HL/NHLBI NIH HHS/United States
- P01 HL054710/HL/NHLBI NIH HHS/United States
- P01 HL054710-050002/HL/NHLBI NIH HHS/United States
- R29 AG014473/AG/NIA NIH HHS/United States
- R01 AG014473-13/AG/NIA NIH HHS/United States
- P01 AG030128/AG/NIA NIH HHS/United States
- R01 AG014473/AG/NIA NIH HHS/United States
- R01 HL050784-15/HL/NHLBI NIH HHS/United States
- HL054710/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous