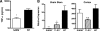The immune adaptor molecule SARM modulates tumor necrosis factor alpha production and microglia activation in the brainstem and restricts West Nile Virus pathogenesis - PubMed (original) (raw)
The immune adaptor molecule SARM modulates tumor necrosis factor alpha production and microglia activation in the brainstem and restricts West Nile Virus pathogenesis
Kristy J Szretter et al. J Virol. 2009 Sep.
Abstract
Sterile alpha and HEAT/Armadillo motif (SARM) is a highly conserved Toll/interleukin-1 receptor (TIR)-containing adaptor protein that is believed to negatively regulate signaling of the pathogen recognition receptors Toll-like receptor 3 (TLR3) and TLR4. To test its physiological function in the context of a microbial infection, we generated SARM(-/-) mice and evaluated the impact of this deficiency on the pathogenesis of West Nile virus (WNV), a neurotropic flavivirus that requires TLR signaling to restrict infection. Although SARM was preferentially expressed in cells of the central nervous system (CNS), studies with primary macrophages, neurons, or astrocytes showed no difference in viral growth kinetics. In contrast, viral replication was increased specifically in the brainstem of SARM(-/-) mice, and this was associated with enhanced mortality after inoculation with a virulent WNV strain. A deficiency of SARM was also linked to reduced levels of tumor necrosis factor alpha (TNF-alpha), decreased microglia activation, and increased neuronal death in the brainstem after WNV infection. Thus, SARM appears to be unique among the TIR adaptor molecules, since it functions to restrict viral infection and neuronal injury in a brain region-specific manner, possibly by modulating the activation of resident CNS inflammatory cells.
Figures
FIG. 1.
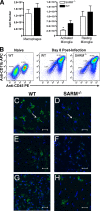
Expression and generation of SARM knockout mice. (A) Schematic of the SARM locus and targeting cassette. Exons 1 through 6 of SARM are represented by gray boxes, and the fragments included in the targeting construct by a heavy black line. The locations of external probes are shown, as are the restriction sites used for screening. S, SphI. (B) A representative image of the RT-PCR analysis of SARM mRNA from whole organs, specific brain regions, and primary cells derived from wild-type C57BL/6 mice is shown. The SARM transcript, at approximately 650 bp, is indicated; a 519-bp fragment of β-actin was amplified in parallel as a control. BM Mac, bone marrow-derived macrophages. (C) A representative image of the RT-PCR analysis of SARM−/− and wild-type mouse mRNA is shown. SARM and β-actin mRNA were amplified from equivalent amounts of mRNA derived from wild-type, SARM+/−, and SARM−/− astrocytes. (D) A representative image of RT-PCR analysis of SARM mRNA in macrophages activated with LPS and TNF-α or infected with WNV is shown. Cerebral cortex mRNA was used as a positive control. M, molecular size ladder.
FIG. 2.
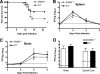
Survival and viral burden analysis for wild-type and SARM−/− mice following WNV challenge. (A) Age-matched 8- to 12-week-old wild-type or SARM−/− mice were inoculated with 102 PFU of WNV-NY by footpad injection and monitored for 21 days. Survival differences were statistically significant (39 SARM−/− and 43 wild-type mice, P < 0.03). (B, C) Viral burden was determined by plaque assay in the spleen (B) and brain (C) on days 2, 4, 6, and 8 after subcutaneous WNV-NY infection. (D) Eight- to 12-week-old mice were inoculated with 101 PFU of WNV-MAD by intracranial injection, and viral burdens in brain and spinal cord were determined by plaque assay on day 6 after infection. Viral burden data are expressed as log10 PFU per gram ± the standard error of the mean for 6 to 10 mice per time point. The dotted lines denote the limit of detection of the viral plaque assay.
FIG. 3.
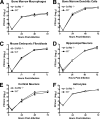
SARM does not have a direct antiviral effect against WNV in primary cells. Primary cells derived from SARM−/− and wild-type mice (described in Materials and Methods) were infected with a multiplicity of infection of 0.001, and virus replication was measured at 6, 24, 48, and 72 h postinfection by viral plaque assay. Multistep growth curves showed no difference in viral yields between SARM−/− and wild-type (A) macrophages, (B) dendritic cells, (C) fibroblasts, (D) hippocampal neurons, (E) cortical neurons, or (F) astrocytes. Viral burden data are expressed as log10 PFU per ml ± the standard error of the mean for triplicate samples from two independent experiments.
FIG. 4.
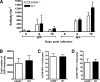
Lymphocyte responses after WNV-NY infection in SARM−/− mice are intact. (A) Wild-type and SARM−/− mice were inoculated with 102 PFU of WNV-NY by footpad injection, and serum samples collected on days 6, 8, and 10 after subcutaneous WNV-NY infection were assayed for WNV E-specific IgM and IgG. Titers are expressed as the reciprocal serum dilution that was three standard deviations above background. Differences were not statistically significant (P > 0.2). (B to D) Wild-type and SARM−/− mice were inoculated with 102 PFU of WNV-NY by footpad injection, and brains were harvested on day 8. Leukocytes were isolated by Percoll gradient centrifugation; stimulated ex vivo with NS4B WNV peptide; stained for CD3, CD8, and intracellular IFN-γ or TNF-α; and analyzed by flow cytometry. (B) The total number of brain CD8+ T cells was determined by the percentage of CD3+ CD8+ cells multiplied by the total cell count. The percentage of CD3+ CD8+ T cells positive for intracellular IFN-γ (C) or TNF-α (D) is indicated. Differences were not statistically significant (P > 0.9). Error bars show standard errors of the means.
FIG. 5.
A deficiency of SARM affects the TNF-α response during WNV infection. Wild-type, TLR3−/−, or SARM−/− mice were inoculated with 101 PFU of WNV-MAD by intracranial injection, and brains were harvested on day 6. (A) Whole-brain homogenates were assayed for TNF-α by ELISA. Data represent the average results for 8 to 10 mice from at least two independent experiments. (B) Brainstem and cortex regions of brain were harvested and analyzed by quantitative RT-PCR to measure TNF-α mRNA levels. Data represent the average results for 10 mice from at least two independent experiments. Error bars show standard errors of the means. *, P < 0.02; **, P < 0.005; N.S., not significant.
FIG. 6.
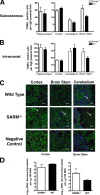
SARM promotes microglia activation during WNV infection. Wild-type or SARM−/− mice were inoculated with 101 PFU of WNV-MAD by intracranial injection, and brains were harvested on day 8. Leukocytes were isolated by Percoll gradient centrifugation, stained for CD11b and CD45, and analyzed by flow cytometry. (A) Expression profiles for activated macrophages (CD11bhigh CD45high), activated microglia (CD11bhigh CD45low), and resting microglia (CD11blow CD45low) were evaluated. Error bars show standard errors of the means. (B) Representative flow cytometry profiles of CD11b and CD45 staining of brain leukocytes from naïve wild-type mice and from wild-type and SARM−/− mice 8 days after WNV infection are shown. (C to H) Representative confocal microscopic images of CD11b (green) and ToPro-3 nuclear staining (blue) of microglia and macrophages in brainstem (C, D), cortex (E, F), and cerebellum (G, H) are shown (*, P < 0.04). White arrows indicate ramified microglia. The scale bar represents ∼15 μm.
FIG. 7.
SARM protects against neuronal death during WNV infection. (A, B) Wild-type or SARM−/− mice were inoculated with either 102 PFU of WNV-NY by footpad injection (A) or 101 PFU of WNV-MAD by intracranial injection (B). Brains were harvested on day 10 or 8, respectively, and were analyzed for neuronal death by using an immunohistochemical apoptosis assay. The number of TUNEL-positive cells was quantified from 10 high-power fields per brain region per mouse for three to five independent mice. Note, fewer TUNEL-positive cells are observed after footpad than after intracranial injection. (C) Representative confocal microscopy images of MAP-2 (green; neuronal cell bodies and dendrites), terminal deoxynucleotidyl transferase (red; TUNEL, nuclear), and ToPro-3 (blue; nuclear) fluorescence staining of neurons in different brain regions in uninfected (negative control) or WNV-MAD-infected wild-type and SARM−/− mice. Arrows indicate TUNEL-positive cells. The scale bar represents ∼15 μm. Data are representative of results for three independent mice. (D) Cerebral cortex and brainstem regions of the brain were harvested and analyzed by quantitative RT-PCR for levels of WNV RNA. Data were normalized for tissue 18S RNA levels and represent the average results for 10 mice from at least two independent experiments. Error bars show standard errors of the means.
Similar articles
- SARM modulates MyD88-mediated TLR activation through BB-loop dependent TIR-TIR interactions.
Carlsson E, Ding JL, Byrne B. Carlsson E, et al. Biochim Biophys Acta. 2016 Feb;1863(2):244-53. doi: 10.1016/j.bbamcr.2015.11.021. Epub 2015 Nov 22. Biochim Biophys Acta. 2016. PMID: 26592460 - The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system.
Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M Jr, Diamond MS. Szretter KJ, et al. J Virol. 2010 Dec;84(23):12125-38. doi: 10.1128/JVI.01026-10. Epub 2010 Sep 29. J Virol. 2010. PMID: 20881045 Free PMC article. - SARM is required for neuronal injury and cytokine production in response to central nervous system viral infection.
Hou YJ, Banerjee R, Thomas B, Nathan C, García-Sastre A, Ding A, Uccellini MB. Hou YJ, et al. J Immunol. 2013 Jul 15;191(2):875-83. doi: 10.4049/jimmunol.1300374. Epub 2013 Jun 7. J Immunol. 2013. PMID: 23749635 Free PMC article. - Beyond TLR Signaling—The Role of SARM in Antiviral Immune Defense, Apoptosis & Development.
Panneerselvam P, Ding JL. Panneerselvam P, et al. Int Rev Immunol. 2015;34(5):432-44. doi: 10.3109/08830185.2015.1065826. Epub 2015 Aug 13. Int Rev Immunol. 2015. PMID: 26268046 Review. - The Troll in Toll: Mal and Tram as bridges for TLR2 and TLR4 signaling.
Sheedy FJ, O'Neill LA. Sheedy FJ, et al. J Leukoc Biol. 2007 Aug;82(2):196-203. doi: 10.1189/jlb.1206750. Epub 2007 Apr 20. J Leukoc Biol. 2007. PMID: 17449723 Review.
Cited by
- Histone methylation analysis and pathway predictions in chickens after MDV infection.
Luo J, Mitra A, Tian F, Chang S, Zhang H, Cui K, Yu Y, Zhao K, Song J. Luo J, et al. PLoS One. 2012;7(7):e41849. doi: 10.1371/journal.pone.0041849. Epub 2012 Jul 26. PLoS One. 2012. PMID: 22848633 Free PMC article. - Insights into the antiviral immunity against grass carp (Ctenopharyngodon idella) reovirus (GCRV) in grass carp.
Rao Y, Su J. Rao Y, et al. J Immunol Res. 2015;2015:670437. doi: 10.1155/2015/670437. Epub 2015 Feb 9. J Immunol Res. 2015. PMID: 25759845 Free PMC article. Review. - Sarm1 knockout prevents type 1 diabetic bone disease in females independent of neuropathy.
Brazill JM, Shen IR, Craft CS, Magee KL, Park JS, Lorenz M, Strickland A, Wee NK, Zhang X, Beeve AT, Meyer GA, Milbrandt J, DiAntonio A, Scheller EL. Brazill JM, et al. JCI Insight. 2024 Jan 4;9(4):e175159. doi: 10.1172/jci.insight.175159. JCI Insight. 2024. PMID: 38175722 Free PMC article. - Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology.
Chen CY, Lin CW, Chang CY, Jiang ST, Hsueh YP. Chen CY, et al. J Cell Biol. 2011 May 16;193(4):769-84. doi: 10.1083/jcb.201008050. Epub 2011 May 9. J Cell Biol. 2011. PMID: 21555464 Free PMC article. - CX3CR1 RNAi inhibits hypoxia-induced microglia activation via p38MAPK/PKC pathway.
Liu Y, Zhao T, Yang Z, Li Q. Liu Y, et al. Int J Exp Pathol. 2014 Apr;95(2):153-7. doi: 10.1111/iep.12065. Int J Exp Pathol. 2014. PMID: 24628787 Free PMC article.
References
- Beasley, D. W., C. T. Davis, M. Whiteman, B. Granwehr, R. M. Kinney, and A. D. Barrett. 2004. Molecular determinants of virulence of West Nile virus in North America. Arch. Virol. Suppl. 2004:35-41. - PubMed
- Belinda, L. W., W. X. Wei, B. T. Hanh, L. X. Lei, H. Bow, and D. J. Ling. 2008. SARM: a novel Toll-like receptor adaptor, is functionally conserved from arthropod to human. Mol. Immunol. 45:1732-1742. - PubMed
- Carty, M., R. Goodbody, M. Schroder, J. Stack, P. N. Moynagh, and A. G. Bowie. 2006. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 7:1074-1081. - PubMed
- Cheeran, M. C., S. Hu, W. S. Sheng, A. Rashid, P. K. Peterson, and J. R. Lokensgard. 2005. Differential responses of human brain cells to West Nile virus infection. J. Neurovirol. 11:512-524. - PubMed
- Couillault, C., N. Pujol, J. Reboul, L. Sabatier, J. F. Guichou, Y. Kohara, and J. J. Ewbank. 2004. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat. Immunol. 5:488-494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases