VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo - PubMed (original) (raw)
Comparative Study
VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo
Ina M Wittko et al. J Neurosci. 2009.
Abstract
The generation of new neurons in the olfactory bulb (OB) persists into adulthood and is a multistep process that includes proliferation, fate choice, migration, survival, and differentiation. Neural precursor cells destined to form olfactory interneurons arise in the subventricular zone (SVZ) and migrate along the rostral migratory stream (RMS) to the OB. Recently, some factors classically known from their effects on the vascular system have been found to influence different steps of adult neurogenesis. In the present study, we report a modulatory function for the vascular endothelial growth factor receptor-1 (VEGFR-1) in adult olfactory neurogenesis. We identified expression of VEGFR-1 in GFAP-positive cells within regions involved in neurogenesis of the adult mouse brain. To determine functions for VEGFR-1 in adult neurogenesis, we compared neural progenitor cell proliferation, migration, and differentiation from wild-type and VEGFR-1 signaling-deficient mice (Flt-1TK(-/-) mice). Our data show that VEGFR-1 signaling is involved in the regulation of proliferation of neuronal progenitor cells within the SVZ, migration along the RMS, and in neuronal differentiation and anatomical composition of interneuron subtypes within the OB. RMS migration in Flt-1TK(-/-) mice was altered mainly as a result of increased levels of its ligand VEGF-A, which results in an increased phosphorylation of VEGFR-2 in neuronal progenitor cells within the SVZ and the RMS. This study reveals that proper RMS migration is dependent on endogenous VEGF-A protein.
Figures
Figure 1.
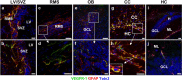
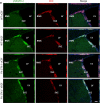
Expression of VEGFR-1 in neurogenic areas of the adult mouse brain. a–j, Sagittal sections of the brain showing VEGFR-1 expression (green) in GFAP+ cells (red), counterstained with DAPI (blue) in different brain areas. a, b, SVZ of the LV. c, Section of adult mouse RMS showing expression of VEGFR-1 (green) throughout the stream in GFAP+ cells. d, Higher magnification of c. e, f, In the adult mouse OB, expression of VEGFR-1 (green) was detected in GFAP+ cells mainly in GCL. f, Higher magnification of e. g, h, Many GFAP+ cells (red) cells in CC coexpress VEGFR-1 (green). h, Higher magnification of g. Inset in g shows an example of clear expression of VEGFR-1 (green) and GFAP (red) in the same cell, nucleus (DAPI, blue). i, j, sections of adult mouse HC with scattered GFAP+ cells (red) expressing VEGFR-1 (green). Scale bars: a, b, f, g, h, j, 20 μm; c, d, e, i, 50 μm. H, Hilus; ML, Molecular layer.
Figure 2.
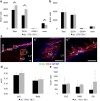
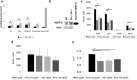
Increased proliferation of subventricular NPCs in _Flt-1TK_−/− mice. a, BrdU immunohistochemistry 3 h after a single bolus of BrdU showed higher number of BrdU+ cells in the SVZ of _Flt-1TK_−/− mice compared with WT. Triple immunofluorescence as shown in c revealed that the increase in BrdU+ cells in the SVZ of _Flt-1TK_−/− compared with WT-mice lies within the DCX+ cell population. In contrast, BrdU+ cells expressing neither GFAP nor DCX were decreased in the _Flt-1TK_−/− mice compared with controls. *p < 0.05. n = 4 b, No difference was found in the RMS. _Flt-1TK_−/−, n = 4; WT, n = 5. c, Triple immunofluorescence of BrdU (green), DCX (red), and GFAP (blue) in the SVZ and the RMS. c′ and c″ are higher magnifications of the marked areas in c. Scale bar, 30 μm. d, _Flt-1TK_−/− and WT mice showed equal volumes of the SVZ. The area within the RMS in which BrdU cells proliferated was similar between both genotypes. e, No significant difference in the number of TUNEL-labeled cells was found between _Flt-1TK_−/− and WT mice. n = 3.
Figure 3.
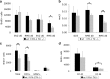
VEGFR-1-signaling deficient _Flt-1TK_−/− mice display a strong change in migration behavior of NPCs in the RMS. _Flt-1TK_−/− and WT mice were injected with BrdU on 5 consecutive days, and BrdU-labeled cells were analyzed on the last day of BrdU application (d0) and after 6 additional days (d6). a, The RMS of _Flt-1TK_−/− mice showed less BrdU+ cells already at day 0 when compared with WT mice. On day 6, BrdU-marked cells were significantly reduced by 47.97% of the mean. In the SVZ, no quantitative difference in BrdU+ cells between genotypes was detected at either time point. *p < 0.0003. _Flt-1TK_−/−, n = 11; WT, n = 10. b, In _Flt-1TK_−/− mice, the spreading area of labeled cells within the RMS was significantly smaller than in WT mice. There was no difference between both genotypes in SVZ volume. *p < 0.0006; **p < 0.03. c, Confocal analysis of triple immunofluorescence showed that especially the DCX+/BrdU+ neuronal progenitor cell population is reduced in the RMS of _Flt-1TK_−/− mice compared with control. *p < 0.02; **p < 0.03. _Flt-1TK_−/−, n = 11; WT, n = 10. d, The number and density of BrdU+ cells entering the CC was significantly reduced in _Flt-1TK_−/− mice on day 6 (compared with WT mice). *p = 0.034. Day 0: _Flt-1TK_−/−, n = 5; WT, n = 7. Day 6: _Flt-1TK_−/−, n = 4; WT, n = 5.
Figure 4.
SVZ explants from VEGFR-1-signaling-deficient _Flt-1TK_−/− mice show increased migration speed in vitro. SVZ tissue from _Flt-1TK_−/− and WT mice was cultured in Matrigel for 13 d. a, Out-migrating cells form networks of highly compacted cells that express neural markers. Scale bars: a, 500 μm; a′, 100 μm; a′′, 10 μm. b, Cells of SVZ explants derived from _Flt-1TK_−/− have migrated farther than those derived from WT mice at every time point. *p = 0.008; **p = 0.003; ***p < 0.02. c, Cells from _Flt-1TK_−/− SVZ explants migrated more than two times faster than cells from WT SVZ explants. _Flt-1TK_−/−, n = 22; WT, n = 15.
Figure 5.
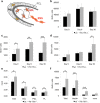
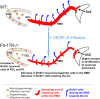
VEGFR-1 deficiency leads to an increased generation of new neurons in the OB resulting in increased OB size. a, Schematic drawing of the migration of NPCs into the olfactory bulb. Cells entering the OB detach from the chains and mainly migrate into the inner GCL. A small percentage migrates through the PL (dark gray) into the periglomerular layer (light gray). b–f, _Flt-1TK_−/− and WT mice were injected with BrdU on 5 consecutive days, and BrdU-labeled cells were analyzed on the last day of BrdU application (Day 0), after 6 (Day 6), and 30 additional days (Day 30). b, The GCL of _Flt-1TK_−/− mice showed higher numbers of BrdU+ cells than the GCL of control animals, but this difference did not reach statistical significance. n = 5. c, In _Flt-1TK_−/− mice, a significantly higher number of BrdU-labeled cells migrate through the PL. *p < 0.05; **p = 0.006; ***p < 0.0002. d, The wave of BrdU reached the PGL at day 30. Then the number of BrdU+ cells has more than doubled in the PGL of _Flt-1TK_−/− than in WT mice. *p = 0.017; n = 5. e, Volumetric analysis of the OB revealed that _Flt-1TK_−/− mice had an increased volume of the OB. The volume of every layer was significantly higher than in control animals. The PGL showed the highest increase with 157.02% of the PGL volume in controls. Total, *p = 0.0008; GCL, *p = 0.045; PGL, *p = 0.0003; PL, *p = 0.018. n = 12. f, Confocal analysis of triple immunofluorescence using the markers anti-BrdU, NeuN, and anti-tyrosine hydroxylase revealed a significant increase in the number of newly formed neurons marked by NeuN in the PGL of _Flt-1TK_−/− compared with WT mice. In addition, the number of cells expressing tyrosine hydroxylase (TH), a marker of dopaminergic neurons, was significantly higher in the PGL of _Flt-1TK_−/− than in controls. *p = 0.02; **p = 0.047; ***p = 0.014. _Flt-1TK_−/−, n = 5; WT, n = 4.
Figure 6.
VEGF-A infusion in WT mice is sufficient to mimic the migration phenotype observed in _Flt-1TK_−/− mice. a, VEGF-A protein of brain tissue of _Flt-1TK_−/− and WT mice was quantified by ELISA. _Flt-1TK_−/− mice display higher levels of VEGF-A protein in different brain areas compared with controls. Pool, Tissue from three brains. Hemisphere, *p = 0.02; cortex, *p = 0.026; striatum + HC, *p = 0.0075. n = 5. b, Western blot for VEGF-A confirms increased amount of VEGF-A protein in _Flt-1TK_−/− mice. Histone 2A serves as loading control. c–e, _Flt-1TK_−/− and control animals received BrdU injections (intraperitoneally) on 5 consecutive days. After the last injection, osmotic minipumps were implanted intracerebroventricularly to deliver VEGF-A or aCSF as control for 6 d. c, Infusion of VEGF-A into the LV of WT mice decreased the number of BrdU-labeled cells in the RMS on day 6 to the level of _Flt-1TK_−/− mice. The number of BrdU-positive cells in _Flt-1TK_−/− mice did not change with VEGF-A infusions. The reduction was mainly in the BrdU+/DCX+ cell population in _Flt-1TK_−/−. Total, *p < 0.001; DCX, *p < 0.01. The number of BrdU+ cells in the RMS of _Flt-1TK_−/− mice did not change with VEGF-A infusions. *p = 0.0009; **p = 0.0001; ***p = 0.0008. d, VEGF-A infusions did not change the number of BrdU+ cells detected in the SVZ on day 6. e, VEGF-A infusion was sufficient to reduce the spreading area of migrating labeled BrdU cells within the RMS in WT mice to the size off this area measured in _Flt-1TK_−/− mice. *p < 0.05. Flt-1TK −/− (aCSF), n = 3; _Flt-1TK_−/− (VEGF), n = 5; WT (aCSF), n = 6; WT (VEGF), n = 7. Ctx, Cortex; H, hemisphere; STR, striatum.
Figure 7.
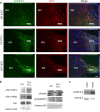
Phosphorylation of VEGFR-2 is increased in NPCs of the SVZ of _Flt-1TK_−/− mice and mice that intracerebrally received VEGF-A. Sagittal sections of the brain showing VEGFR-2 phosphorylation (green) in DCX+ cells (red) counterstained with DAPI (blue) in the aSVZ of the LV. VEGFR-2 is phosphorylated particularly in DCX+ cells of aSVZ and the RMS. The intensity of phospho-VEGFR-2 immunoreactivity is much greater in the aSVZ and RMS of _Flt-1TK_−/− mice and in mice that received VEGF-A compared with WT mice. Scale bar, 200 μm.
Figure 8.
Changes in phosphorylation in NPCs of the RMS/OB of _Flt-1TK_−/− mice or mice that intracerebrally received VEGF-A. a, Sagittal sections of the brain showing VEGFR-2 phosphorylation (green) in DCX+ cells (red) counterstained with DAPI (blue) in the RMS at the entry point to the OB. VEGFR-2 is phosphorylated particularly in DCX+ cells of the RMS. Phosphorylation is abolished in cells that entered the GCL. DCX expression persists in NPCs within the OB. The intensity of p-VEGFR-2 immunoreactivity is much greater in the RMS of _Flt-1TK_−/− mice and in mice that received VEGF-A compared with WT mice. Scale bar, 40 μm. b, Western blot confirming the increased phosphorylation of VEGFR-2 on tyrosine 996 (Y996) and 951 (Y951). VEGFR-2 total protein was barely changed. Phosphorylation of p38MAPK was decreased in _Flt-1TK_−/−, whereas Paxillin and FAK phosphorylation was enhanced. c, Mice that received VEGF-A in a single injection show increased levels of p-VEGFR-2 in forebrain lysates. Histone 2A serves as loading control.
Figure 9.
Schematic drawing of the differences observed in the steps of neurogenesis between WT and _Flt-1TK_−/− mice. Olfactory neurons derive from NPCs of the SVZ. _Flt-1TK_−/− display higher proliferation rates of DCX+ cells in the aSVZ. Our data point toward a faster migration, which accounts for the fact that, in _Flt-1TK_−/− at day 6, there are already fewer BrdU-labeled NPCs in the RMS than in WT mice. In addition, during migration, the spreading of BrdU-labeled cells in _Flt-1Tk_−/− is smaller than in controls. Once in the OB, the cells detach and migrate as single cells into the different layers of the OB. In _Flt-1TK_−/− mice, significantly more cells migrate through the PL into the PGL than in WT mice. In addition, more neurons form in the PGL of _Flt-1TK_−/− and especially more neurons of a dopaminergic subtype when compared with WT mice. As a result of their constant increase in neurogenesis the OB of the _Flt-1TK_−/− mice is bigger in size than that of controls. Infusion of VEGF-A into the LV of WT mice during the migration period was sufficient to induce the migration phenotype observed in _Flt-1TK_−/− mice.
Similar articles
- Intrinsic Neuronal Activity during Migration Controls the Recruitment of Specific Interneuron Subtypes in the Postnatal Mouse Olfactory Bulb.
Bugeon S, Haubold C, Ryzynski A, Cremer H, Platel JC. Bugeon S, et al. J Neurosci. 2021 Mar 24;41(12):2630-2644. doi: 10.1523/JNEUROSCI.1960-20.2021. Epub 2021 Feb 3. J Neurosci. 2021. PMID: 33536198 Free PMC article. - Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling.
Garzotto D, Giacobini P, Crepaldi T, Fasolo A, De Marchis S. Garzotto D, et al. J Neurosci. 2008 Jun 4;28(23):5901-9. doi: 10.1523/JNEUROSCI.1083-08.2008. J Neurosci. 2008. PMID: 18524894 Free PMC article. - Meis2 is a Pax6 co-factor in neurogenesis and dopaminergic periglomerular fate specification in the adult olfactory bulb.
Agoston Z, Heine P, Brill MS, Grebbin BM, Hau AC, Kallenborn-Gerhardt W, Schramm J, Götz M, Schulte D. Agoston Z, et al. Development. 2014 Jan;141(1):28-38. doi: 10.1242/dev.097295. Epub 2013 Nov 27. Development. 2014. PMID: 24284204 - Relationship between Blood Vessels and Migration of Neuroblasts in the Olfactory Neurogenic Region of the Rodent Brain.
Martončíková M, Alexovič Matiašová A, Ševc J, Račeková E. Martončíková M, et al. Int J Mol Sci. 2021 Oct 25;22(21):11506. doi: 10.3390/ijms222111506. Int J Mol Sci. 2021. PMID: 34768936 Free PMC article. Review. - Olfactory bulb neurogenesis depending on signaling in the subventricular zone.
Chen Y, Ren P, He X, Yan F, Gu R, Bai J, Zhang X. Chen Y, et al. Cereb Cortex. 2023 Nov 4;33(22):11102-11111. doi: 10.1093/cercor/bhad349. Cereb Cortex. 2023. PMID: 37746807 Review.
Cited by
- Autocrine VEGF drives neural stem cell proximity to the adult hippocampus vascular niche.
Dause TJ, Denninger JK, Osap R, Walters AE, Rieskamp JD, Kirby ED. Dause TJ, et al. Life Sci Alliance. 2024 Apr 17;7(7):e202402659. doi: 10.26508/lsa.202402659. Print 2024 Jul. Life Sci Alliance. 2024. PMID: 38631901 Free PMC article. - Role of Maternal Immune Factors in Neuroimmunology of Brain Development.
Mohebalizadeh M, Babapour G, Maleki Aghdam M, Mohammadi T, Jafari R, Shafiei-Irannejad V. Mohebalizadeh M, et al. Mol Neurobiol. 2024 Dec;61(12):9993-10005. doi: 10.1007/s12035-023-03749-2. Epub 2023 Dec 7. Mol Neurobiol. 2024. PMID: 38057641 Review. - Migratory Response of Cells in Neurogenic Niches to Neuronal Death: The Onset of Harmonic Repair?
Geribaldi-Doldán N, Carrascal L, Pérez-García P, Oliva-Montero JM, Pardillo-Díaz R, Domínguez-García S, Bernal-Utrera C, Gómez-Oliva R, Martínez-Ortega S, Verástegui C, Nunez-Abades P, Castro C. Geribaldi-Doldán N, et al. Int J Mol Sci. 2023 Apr 1;24(7):6587. doi: 10.3390/ijms24076587. Int J Mol Sci. 2023. PMID: 37047560 Free PMC article. Review. - Gut brain interaction theory reveals gut microbiota mediated neurogenesis and traditional Chinese medicine research strategies.
Zhang C, Xue P, Zhang H, Tan C, Zhao S, Li X, Sun L, Zheng H, Wang J, Zhang B, Lang W. Zhang C, et al. Front Cell Infect Microbiol. 2022 Dec 8;12:1072341. doi: 10.3389/fcimb.2022.1072341. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36569198 Free PMC article. Review. - Alzheimer's Disease: Challenges and a Therapeutic Opportunity to Treat It with a Neurotrophic Compound.
Baazaoui N, Iqbal K. Baazaoui N, et al. Biomolecules. 2022 Oct 2;12(10):1409. doi: 10.3390/biom12101409. Biomolecules. 2022. PMID: 36291618 Free PMC article. Review.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Beck H, Acker T, Püschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol. 2002;61:339–350. - PubMed
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous