Visual field maps, population receptive field sizes, and visual field coverage in the human MT+ complex - PubMed (original) (raw)
Visual field maps, population receptive field sizes, and visual field coverage in the human MT+ complex
Kaoru Amano et al. J Neurophysiol. 2009 Nov.
Abstract
Human neuroimaging experiments typically localize motion-selective cortex (MT+) by contrasting responses to stationary and moving stimuli. It has long been suspected that MT+, located on the lateral surface at the temporal-occipital (TO) boundary, contains several distinct visual field maps, although only one coarse map has been measured. Using a novel functional MRI model-based method we identified two maps-TO-1 and TO-2-and measured population receptive field (pRF) sizes within these maps. The angular representation of the first map, TO-1, has a lower vertical meridian on its posterior side at the boundary with the lateral-occipital cortex (i.e., the LO-2 portion). The angular representation continues through horizontal to the upper vertical meridian at the boundary with the second map, TO-2. The TO-2 angle map reverses from upper to lower visual field at increasingly anterior positions. The TO maps share a parallel eccentricity map in which center-to-periphery is represented in the ventral-to-dorsal direction; both maps have an expanded foveal representation. There is a progressive increase in the pRF size from V1/2/3 to LO-1/2 and TO-1/2, with the largest pRF sizes in TO-2. Further, within each map the pRF size increases as a function of eccentricity. The visual field coverage of both maps extends into the ipsilateral visual field, with larger sensitivity to peripheral ipsilateral stimuli in TO-2 than that in TO-1. The TO maps provide a functional segmentation of human motion-sensitive cortex that enables a more complete characterization of processing in human motion-selective cortex.
Figures
Fig. 1.
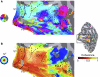
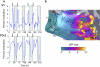
Temporal–occipital (TO) visual field maps in a right hemisphere. The data shown are from one subject (S2) with a particularly clear visual field map organization. The data are shown on the subject's own inflated cortical surface. The inset at the right shows the motion-selective middle temporal area (MT+) localizer responses; the black rectangle indicates the region of posterior temporal–occipital cortex that is shown in a more detailed view in the left panels. A: angle maps. The legend shows the relationship between color and the most effective stimulus angle. The temporal–occipital cortex (lower to upper vertical meridian, TO-1) has a peak response to angles near the lower vertical meridian on its posterior side at the boundary with the lateral occipital cortex (upper to lower vertical meridian, LO-2). The angular representation of TO-1 continues through horizontal toward the upper vertical meridian at the boundary with TO-2 and the TO-2 angle map reverses. Light and dark shaded regions of the cortical surface indicate gyri and sulci locations, respectively. B: eccentricity maps. The legend shows the relationship between color and most effective eccentricity. Two distinct foveal confluences are present. The foveal representation for V1/V2/V3/LO-1/2 is posterior (left) compared with the TO-1/2 foveal representation. In the TO-1/2 maps, eccentricity increases from ventral to dorsal.
Fig. 2.
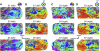
Temporal–occipital visual field maps in 6 additional hemispheres. The data shown are from 6 hemispheres chosen to illustrate the variation between subjects (all subjects are shown in Supplemental Figs. S1, S2, S5, and S6). Each data set is shown on the subject's own inflated cortical surface. Other details are as in Fig. 1. Anterior border of some TO-2 maps (e.g., S1 left and S5 left) represents the horizontal rather than the lower vertical meridian. TO-1/TO-2 fovea were not separated from V1/V2/V3/LO-1/2 for some hemispheres (e.g., S3 left, right and S5 left). See Table 1 for detail of the variance with respect to several map landmarks.
Fig. 3.
Average angle and eccentricity maps. The individual maps were transformed into a common atlas space and averaged across all 14 hemispheres. A: averaged angle maps. Angle reversals are evident at the boundaries. Peak angles in LO-1/2 and TO-1/2 fall within both the lower and upper visual fields. B: averaged eccentricity maps. TO-1 and TO-2 share a foveal representation that is distinct from that of LO-1/2. LO-1 mostly represents the foveal region. The angle and eccentricity maps are not orthogonal in LO-2 and TO-1/2. Approximate length of the atlas of visual cortical areas V1–V3 (5 cm) was obtained by averaging the cortical distance of V1 representing horizontal meridian. Approximate length of the atlas of LO and TO (2.5 cm) was obtained by averaging all 5 borders of maps across all subjects.
Fig. 4.
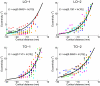
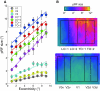
Cortical magnification in the LO and TO maps. The most effective eccentricity is shown as a function of cortical distance. Separate panels plot the functions for LO-1/2 and TO-1/2. The data are aligned at the 10° eccentricity point. For each hemisphere, eccentricity was measured along 4 isoangle lines, derived from the atlas procedure (see
methods
for details), and averaged across these lines. Cortical distances are grouped into bins and the average eccentricity within each bin was computed. Each color represents a different subject and each symbol type represents the left (+) or right (×) hemisphere. The smooth curve is the best (least-squares) fit to the data; the equation of the fit is shown in each panel where E is the eccentricity (degrees) and D is the cortical distance (mm).
Fig. 5.
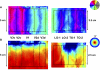
Population receptive field (pRF) size map in a right hemisphere (S2). A: the time-series responses to a rotating wedge stimulus with inserted blanks (mean-luminance) from a region of interest in V1 (top) and TO-2 (bottom) are shown. The locations of these regions are shown by the circles on the inflated cortical surface (B). The vertical arrows indicate the strongest functional magnetic resonance imaging (fMRI) response elicited by the same wedge orientation. Light blue regions indicate the mean-luminance blocks. The 2 cortical locations responded maximally to roughly similar wedge positions. In V1, little fMRI modulation is observed by the insertion of mean-luminance blocks. In TO-2, every insertion of mean-luminance blocks causes a drop in the blood oxygen level–dependent modulation, demonstrating that TO-2 is responsive to all wedge orientations. These differences are explained mainly by differences in the pRF size (Dumoulin and Wandell 2008). B: the pRF size is shown on the subject's inflated cortical surface. The color overlay shows the estimated pRF size (σ). There is a progressive increase in pRF size from V1 to TO-2. The pRF size in V1/V2/V3/LO-1 is <3°, whereas the size in TO-1/2 exceeds 10°.
Fig. 6.
The relation between pRF size and eccentricity and the average pRF size map. A: averaged pRF size (σ) as a function of eccentricity for all identified visual field maps. The pRF size increase from V1/2/3 to LO-1/2 and TO-1/2. Within each visual field map, pRF size increases linearly with eccentricity. B: the individual pRF maps were averaged across all subjects in a common atlas space. The color overlay shows the pRF size (σ). Both the increase in pRF size across maps and the eccentricity-dependent increase in pRF size within each map (from bottom to top) are observable.
Fig. 7.
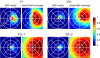
Averaged pRF center distribution and visual field coverage. The separate panels show measurements from V1, V3v, TO-1, and TO-2. The left panels show the distribution of pRF centers; the right panels incorporate the pRF sizes in the computation of the visual field coverage (see
methods
for details). The data are combined across all subjects and hemispheres. The data from the right hemisphere were converted into left-hemisphere format to pool across both left and right hemispheres. As expected, the V1 and V3v coverage is mainly confined to the contralateral hemifield and contralateral upper quadrant, respectively. The visual field coverage in TO-1 and TO-2 spans the entire contralateral hemifield but extends significantly into the ipsilateral visual field. The ipsilateral extension of TO-2 exceeds that of TO-1, mainly because of the larger pRF sizes; the pRF center distributions are similar. In individual subjects, the TO-1/2 visual field coverage also spans the entire contralateral hemifield.
Fig. 8.
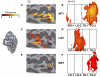
The relationship between visual field maps and conventional functional localizers. The responses for subject S2 (A, C, E) and averaged responses from 14 hemispheres (B, D, F) are shown. The inset at the left shows a posterior view of the subject's inflated cortical surface. The black outline indicates the region shown in A, C, and E. The top panels (A and B) show that the strongest responses to the MT+ localizer coincide with TO-1 and TO-2 (coherence >0.6 for S2 and coherence >0.4 for the average). The middle panels (C and D) show that the strongest responses to the lateral–occipital complex (LOC) localizer coincide with LO maps, in particular LO-2, but not the TO maps (coherence >0.4 for S2 and the average). The bottom panels (E and F) show that the strongest responses to the MST localizer is confined mainly to TO-2 (coherence >0.4 for S2 and coherence >0.3 for the average). The response to the medial superior temporal area (MST) localizer was weak and therefore the threshold for the average response may is lowered. The presented data are highly significant (P < 0.01, corrected).
Fig. 9.
A model of the sequence of maps on the lateral surface. Each colored region represents a visual field map on the lateral surface. The black lines indicate the horizontal isoangle contours of each map; that is, the region most powerfully driven by signals along the horizontal meridian. The white line indicates the isoeccentricity contour (6°) for each map. The model is based on individual subject data (S2).
Similar articles
- Retinotopic organization of human ventral visual cortex.
Arcaro MJ, McMains SA, Singer BD, Kastner S. Arcaro MJ, et al. J Neurosci. 2009 Aug 26;29(34):10638-52. doi: 10.1523/JNEUROSCI.2807-09.2009. J Neurosci. 2009. PMID: 19710316 Free PMC article. - Relating retinotopic and object-selective responses in human lateral occipital cortex.
Sayres R, Grill-Spector K. Sayres R, et al. J Neurophysiol. 2008 Jul;100(1):249-67. doi: 10.1152/jn.01383.2007. Epub 2008 May 7. J Neurophysiol. 2008. PMID: 18463186 Free PMC article. - Two retinotopic visual areas in human lateral occipital cortex.
Larsson J, Heeger DJ. Larsson J, et al. J Neurosci. 2006 Dec 20;26(51):13128-42. doi: 10.1523/JNEUROSCI.1657-06.2006. J Neurosci. 2006. PMID: 17182764 Free PMC article. - Visual field maps in human cortex.
Wandell BA, Dumoulin SO, Brewer AA. Wandell BA, et al. Neuron. 2007 Oct 25;56(2):366-83. doi: 10.1016/j.neuron.2007.10.012. Neuron. 2007. PMID: 17964252 Review. - Human V4 and ventral occipital retinotopic maps.
Winawer J, Witthoft N. Winawer J, et al. Vis Neurosci. 2015 Jan;32:E020. doi: 10.1017/S0952523815000176. Vis Neurosci. 2015. PMID: 26241699 Free PMC article. Review.
Cited by
- Functional localization of visual motion area FST in humans.
Wen P, Ezzo R, Thompson LW, Rosenberg A, Landy MS, Rokers B. Wen P, et al. bioRxiv [Preprint]. 2024 Sep 20:2024.09.19.614014. doi: 10.1101/2024.09.19.614014. bioRxiv. 2024. PMID: 39345389 Free PMC article. Preprint. - The Role of Population Receptive Field Sizes in Higher-Order Visual Dysfunction.
Elul D, Levin N. Elul D, et al. Curr Neurol Neurosci Rep. 2024 Dec;24(12):611-620. doi: 10.1007/s11910-024-01375-6. Epub 2024 Sep 12. Curr Neurol Neurosci Rep. 2024. PMID: 39266871 Free PMC article. Review. - Rethinking simultaneous suppression in visual cortex via compressive spatiotemporal population receptive fields.
Kupers ER, Kim I, Grill-Spector K. Kupers ER, et al. Nat Commun. 2024 Aug 11;15(1):6885. doi: 10.1038/s41467-024-51243-7. Nat Commun. 2024. PMID: 39128923 Free PMC article. - Brain representations of motion and position in the double-drift illusion.
Steinberg NJ, Roth ZN, Movshon JA, Merriam E. Steinberg NJ, et al. Elife. 2024 May 29;13:e76803. doi: 10.7554/eLife.76803. Elife. 2024. PMID: 38809774 Free PMC article. - Sustained attention operates via dissociable neural mechanisms across different eccentric locations.
Phangwiwat T, Phunchongharn P, Wongsawat Y, Chatnuntawech I, Wang S, Chunharas C, Sprague TC, Woodman GF, Itthipuripat S. Phangwiwat T, et al. Sci Rep. 2024 May 16;14(1):11188. doi: 10.1038/s41598-024-61171-7. Sci Rep. 2024. PMID: 38755251 Free PMC article.
References
- Allman JM, Kaas JH. A representation of the visual field in the caudal third of the middle tempral gyrus of the owl monkey (Aotus trivirgatus). Brain Res 31: 85–105, 1971 - PubMed
- Ashburner J, Friston K. Rigid body registration. In: Human Brain Function ( 2nd ed.), edited by Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Friston KJ, Price CJ, Zeki S, Ashburner J, Penny WD. San Diego, CA: Academic Press/Elsevier Science, 2003, p. 635–654
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med 30: 161–173, 1993 - PubMed
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol 296: 462–495, 1990 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources