Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa - PubMed (original) (raw)
Review
Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa
S A Khader et al. Mucosal Immunol. 2009 Sep.
Abstract
T helper type 17 (Th17) cells are a distinct lineage of T cells that produce the effector molecules IL-17, IL-17F, IL-21, and IL-22. Although the role of Th17 cells in autoimmunity is well documented, there is growing evidence that the Th17 lineage and other interleukin (IL)-17-producing cells are critical for host defense against bacterial, fungal, and viral infections at mucosal surfaces. Here we summarize recent progress in our understanding of the function of IL-17-producing cells as a bridge between innate and adaptive immunity against infectious diseases at the mucosa.
Figures
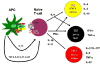
Figure 1. Current understanding of T-helper cell differentiation
Naïve CD4+ T cells, after activation by signaling via the T-cell Receptor and co-stimulatory molecules such as CD28 and Cytotoxic T lymphocyte-associated protein 4 (CTLA-4), can differentiate into three lineages of effector T helper(Th) cells namely Th1, Th2 or Th17 cells. Th subsets produce different cytokines and have distinct immunoregulatory functions. Th2 cells produce IL-4, IL-5 and IL-10 and promote immunity against parasites such as helminths. Th1 cells produce IFNγ and TNF-α and provide immunity against intracellular pathogens. Th17 cells produce the cytokines IL-17/IL-17F, IL-22, IL-21 and TNF-α and primarily promote immunity against extracellular pathogens.
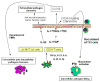
Figure 2. Role of Th17 cytokines in protective immunity at the mucosa
Infection-induced IL-17 and IL-22 can be produced by several immune cells found in mucosal sites. A critical likely target of IL-17 and IL-22 is the mucosal epithelium where IL-17 augments G-CSF and CXC chemokine production resulting in recruitment of neutrophils that contribute to bacterial and fungal clearance at mucosal sites. IL-22 along with IL-17 also augments antimicrobial peptides and epithelial repair function important for control of extracellular fungal pathogens. In the setting of vaccine-induced immunity, Th17 cells can induce the production of ligands for CXCR3 and augment the recruitment of IFNγ producing Th1 cells to control intracellular pathogen growth.
Similar articles
- Th17 cells and mucosal host defense.
Aujla SJ, Dubin PJ, Kolls JK. Aujla SJ, et al. Semin Immunol. 2007 Dec;19(6):377-82. doi: 10.1016/j.smim.2007.10.009. Epub 2007 Nov 28. Semin Immunol. 2007. PMID: 18054248 Free PMC article. Review. - Th17 cytokines and mucosal immunity.
Dubin PJ, Kolls JK. Dubin PJ, et al. Immunol Rev. 2008 Dec;226:160-71. doi: 10.1111/j.1600-065X.2008.00703.x. Immunol Rev. 2008. PMID: 19161423 Review. - The biological functions of T helper 17 cell effector cytokines in inflammation.
Ouyang W, Kolls JK, Zheng Y. Ouyang W, et al. Immunity. 2008 Apr;28(4):454-67. doi: 10.1016/j.immuni.2008.03.004. Immunity. 2008. PMID: 18400188 Free PMC article. Review. - Th17 cells in mucosal immunity and tissue inflammation.
Kolls JK. Kolls JK. Semin Immunopathol. 2010 Mar;32(1):1-2. doi: 10.1007/s00281-010-0198-8. Epub 2010 Feb 25. Semin Immunopathol. 2010. PMID: 20182729 Free PMC article. - Th17 cytokines and vaccine-induced immunity.
Lin Y, Slight SR, Khader SA. Lin Y, et al. Semin Immunopathol. 2010 Mar;32(1):79-90. doi: 10.1007/s00281-009-0191-2. Epub 2010 Jan 30. Semin Immunopathol. 2010. PMID: 20112107 Free PMC article. Review.
Cited by
- IL-17A-mediated neutrophil recruitment limits expansion of segmented filamentous bacteria.
Flannigan KL, Ngo VL, Geem D, Harusato A, Hirota SA, Parkos CA, Lukacs NW, Nusrat A, Gaboriau-Routhiau V, Cerf-Bensussan N, Gewirtz AT, Denning TL. Flannigan KL, et al. Mucosal Immunol. 2017 May;10(3):673-684. doi: 10.1038/mi.2016.80. Epub 2016 Sep 14. Mucosal Immunol. 2017. PMID: 27624780 Free PMC article. - The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review.
Punt S, Langenhoff JM, Putter H, Fleuren GJ, Gorter A, Jordanova ES. Punt S, et al. Oncoimmunology. 2015 Mar 6;4(2):e984547. doi: 10.4161/2162402X.2014.984547. eCollection 2015 Feb. Oncoimmunology. 2015. PMID: 25949881 Free PMC article. Review. - Vitamin D deficiency: protective against enteric infection?
Christakos S. Christakos S. Am J Physiol Gastrointest Liver Physiol. 2012 Dec 15;303(12):G1297-8. doi: 10.1152/ajpgi.00405.2012. Epub 2012 Nov 1. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 23125157 Free PMC article. No abstract available. - The Essentiality of Arachidonic Acid in Infant Development.
Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N Jr. Hadley KB, et al. Nutrients. 2016 Apr 12;8(4):216. doi: 10.3390/nu8040216. Nutrients. 2016. PMID: 27077882 Free PMC article. Review. - HIV-specific Th2 and Th17 responses predict HIV vaccine protection efficacy.
Sauce D, Gorochov G, Larsen M. Sauce D, et al. Sci Rep. 2016 Jun 21;6:28129. doi: 10.1038/srep28129. Sci Rep. 2016. PMID: 27324186 Free PMC article. Clinical Trial.
References
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. - PubMed
- Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL079142/HL/NHLBI NIH HHS/United States
- P50HL084932/HL/NHLBI NIH HHS/United States
- AR054389/AR/NIAMS NIH HHS/United States
- R01 AR054389/AR/NIAMS NIH HHS/United States
- R00AI075106/AI/NIAID NIH HHS/United States
- R00 AI075106/AI/NIAID NIH HHS/United States
- R21 DE018822/DE/NIDCR NIH HHS/United States
- DE018822/DE/NIDCR NIH HHS/United States
- R01HL079142/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources