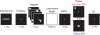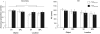Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations - PubMed (original) (raw)
Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations
Massihullah Hamidi et al. Front Integr Neurosci. 2009.
Abstract
A governing assumption about repetitive transcranial magnetic stimulation (rTMS) has been that it interferes with task-related neuronal activity - in effect, by "injecting noise" into the brain - and thereby disrupts behavior. Recent reports of rTMS-produced behavioral enhancement, however, call this assumption into question. We investigated the neurophysiological effects of rTMS delivered during the delay period of a visual working memory task by simultaneously recording brain activity with electroencephalography (EEG). Subjects performed visual working memory for locations or for shapes, and in half the trials a 10-Hz train of rTMS was delivered to the superior parietal lobule (SPL) or a control brain area. The wide range of individual differences in the effects of rTMS on task accuracy, from improvement to impairment, was predicted by individual differences in the effect of rTMS on power in the alpha-band of the EEG ( approximately 10 Hz): a decrease in alpha-band power corresponded to improved performance, whereas an increase in alpha-band power corresponded to the opposite. The EEG effect was localized to cortical sources encompassing the frontal eye fields and the intraparietal sulcus, and was specific to task (location, but not object memory) and to rTMS target (SPL, not control area). Furthermore, for the same task condition, rTMS-induced changes in cross-frequency phase synchrony between alpha- and gamma-band (>40 Hz) oscillations predicted changes in behavior. These results suggest that alpha-band oscillations play an active role cognitive processes and do not simply reflect absence of processing. Furthermore, this study shows that the complex effects of rTMS on behavior can result from biasing endogenous patterns of network-level oscillations.
Keywords: alpha band; electroencephalography; oscillations; rTMS; spatial; transcranial magnetic stimulation; working memory.
Figures
Figure 1
Behavioral task. For each brain area targeted (SPL and S1), subjects performed 192 memory trials (96 location memory and 96 object memory, randomly interleaved). On half the trials, randomly distributed across both memory tasks, a 3-s train of 10-Hz rTMS (30 pulses) coincided with the onset of the delay period.
Figure 2
Behavioral effect of rTMS. A three-way ANOVA (rTMS, brain area, memory task) on accuracy from revealed a main effect of memory task [F(1,14) = 5.22; p < 0.05] and a significant rTMS × memory task interaction [F(1,14) = 4.72; p < 0.05, marked with an asterisk]. The main effect of memory task was driven by the fact that subjects had a higher accuracy in object memory trials compared to location memory trials [t(14) = 2.29; p < 0.05]. The rTMS × memory task interaction was due to an increase in accuracy with rTMS specifically during location working memory trials [t(14) = 2.17; p < 0.05]. All other effects and interactions were non-significant (all _F_-values <1.47). Three-way ANOVA (rTMS, brain area, memory task) on RT showed only a main effect of memory task [F(1,14) = 10.00; p < 0.01]. Subjects were faster at responding to location memory trials compared to object memory trials [t(14) = 3.16; p < 0.01]. There were no other significant main effects or interactions with RT (all _F_-values <2.76).
Figure 3
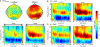
Effect of rTMS on delay-period alpha-band power. (A) During the delay-period there was an increase in power between 10 and 15 Hz for both memory tasks, predominantly over posterior scalp regions. The magnitude of power change between the two memory conditions differed significantly [mean difference across all channels: t(14) = 7.58; p < 10−5], with delay period alpha-band power being significantly greater during object memory trials compared to location memory trials. (B,C) During rTMS trials, there was a brief increase in power at 4–8 Hz associated with the onset of the stimulation train. However, compared to the rTMSabsent trials, there was no significant change in power within the alpha-band range in rTMSpresent trials. On the plots, 0 ms indicates the onset of the 3-s delay period. Time-frequency plots are derived from data obtained at electrode P3, the electrode closest to the SPL TMS target. Topographic plots in (A) represent the mean alpha-band power over the 3-s delay period during rTMSabsent trials.
Figure 4
Negative correlation between SPL rTMS-induced change in alpha-band power and change in accuracy during location working memory trials. Change in alpha-band power (averaged over 8.5–14 Hz) was calculated for each subject as the mean difference in alpha-band power between rTMSabsent and rTMSpresent trials over the entire 3-s delay period. This correlation was observed over a left, posterior cluster of channels, near the location of stimulation, and another right, frontal cluster of channels. For S1 rTMS and for both object memory conditions, there was a trend toward a positive relationship between rTMS-induced change in alpha-band power and change in accuracy (this trend became significant during the second half of the delay period). Data shown is from the electrode immediately below the TMS coil (electrode P3, circled).
Figure 5
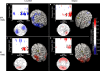
Topographic and source maps of correlations between rTMS-induced change in alpha-band power and accuracy. (A) With SPL rTMS, during location memory trials, there was a negative correlation between rTMS-induced change in alpha-band power and accuracy. These correlations originated from three cortical sources: a large region of cortex extending from the left inferior parietal lobule, along the intraparietal sulcus (BA 39) to the left extrastriate cortex (BA 18), a region covering the left precentral sulcus (BA 6) and superior frontal gyrus, which included the putative frontal eye fields, as well as a region in the right medial temporal lobe corresponding to the hippocampus (not shown). (B) With object memory trials there was a positive correlation between effect of rTMS on alpha-band power and accuracy that was localized to a small area in the right calcarine fissure (not shown). There was also a region showing a negative correlation at the anterior region of the superior frontal sulcus (BA 9). (C,D) With S1 rTMS, for both memory tasks, there was a positive correlation between rTMS-induced change in alpha-band power and rTMS-induced change in accuracy. These correlations were localized predominantly to the precuneus (BA 7), and bilateral occipital cortex (BA 19) for location memory and bilateral occipital cortex (BA 18), right posterior superior temporal gyrus (BA 22) and right anterior superior frontal sulcus (BA 10) for object memory trials. For all task conditions, the correlation was more significant during the late half of the delay period. Topographic and surface plots were calculated after averaging change in power at the time and frequency range indicated by the dotted rectangles on the corresponding time-frequency plots. The standard error of the source estimates of the correlations varied across voxels and task conditions, but had a mean of 0.20 (0.08–0.37). The time-frequency plots were obtained from the electrode shown circled on the topographic plots and were thresholded by time domain as described in the “Materials and Methods” section. Yellow asterisks indicate the target of stimulation with TMS. Colors indicate the _r_-value of the correlation for each condition (r > 0.5 or less than −0.5 correspond to a significance level of p < 0.05).
Figure 6
Topographic and source maps of correlation between rTMS-induced change in oscillatory power and RT. (A,B,D) For three task conditions (SPL/location memory, SPL/object memory and S1/object memory), there was a positive correlation between rTMS-induced change in power at 14–16 Hz and RT. (B,C) For two task conditions (SPL/object memory and S1/location memory), there was a negative correlation between rTMS-induced change in power at 7–10 Hz and RT. Topographic and surface plots were calculated after averaging change in power at the time and frequency range indicated by the dotted rectangles on the corresponding time-frequency plots. The standard error of the source estimates of the correlations varied across voxels and task conditions, but had a mean of 0.21 (0.09–0.42). Time-frequency plots were obtained at the electrodes indicated and were thresholded by time domain as described in the “Materials and Methods” section. The cortical locations of this relationship varied for each task condition and are listed in Table 1.
Figure 7
(A) Raw alpha:gamma phase synchrony for location and object memory in rTMSabsent trials. Pairwise analysis reveals a greater alpha:gamma synchronization in the right posterior electrodes during object memory trials. (B) Change in alpha:gamma phase synchrony with SPL rTMS. (C) Correlation between SPL rTMS-induced change in alpha:gamma phase synchrony and rTMS-induced change in accuracy (r > 0.50 corresponds to p < 0.05). Scatter plots show this relationship for electrode P3 (nearest the location of stimulation). The difference between the correlations in object versus spatial memory trials was significant at right posterior electrodes (_Z_ = 1.83–2.34 for electrodes CP6, TP8 and P6; _p_ = 0.02–0.06) and marginally so for the left posterior electrodes (_Z_ = 1.75–1.87 for electrodes P7, P5 and P3; _p_ = 0.06–0.08). For S1 rTMS, the difference between the correlations for the two task conditions did not reach significance (there were no clusters of channels with _Z_ > 2). All plots are derived from the mean change in PLF during the second half of the delay period. All plots are based on phase synchrony between 10 and 40 Hz oscillations. Analysis of synchrony between 10 and 50 and 10 and 60 Hz reveals qualitatively similar results.
Similar articles
- Theta and Alpha Oscillations during the Retention Period of Working Memory by rTMS Stimulating the Parietal Lobe.
Li S, Jin JN, Wang X, Qi HZ, Liu ZP, Yin T. Li S, et al. Front Behav Neurosci. 2017 Sep 14;11:170. doi: 10.3389/fnbeh.2017.00170. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28959194 Free PMC article. - Using EEG to explore how rTMS produces its effects on behavior.
Johnson JS, Hamidi M, Postle BR. Johnson JS, et al. Brain Topogr. 2010 Jan;22(4):281-93. doi: 10.1007/s10548-009-0118-1. Epub 2009 Nov 14. Brain Topogr. 2010. PMID: 19915972 Free PMC article. - Simultaneously stimulating both brain hemispheres by rTMS in patients with unilateral brain lesions decreases interhemispheric asymmetry.
Zhong Y, Fan J, Wang H, He R. Zhong Y, et al. Restor Neurol Neurosci. 2021;39(6):409-418. doi: 10.3233/RNN-211172. Restor Neurol Neurosci. 2021. PMID: 34334435 - EEG oscillations: From correlation to causality.
Herrmann CS, Strüber D, Helfrich RF, Engel AK. Herrmann CS, et al. Int J Psychophysiol. 2016 May;103:12-21. doi: 10.1016/j.ijpsycho.2015.02.003. Epub 2015 Feb 4. Int J Psychophysiol. 2016. PMID: 25659527 Review. - rTMS in mental health disorders.
Richter K, Kellner S, Licht C. Richter K, et al. Front Netw Physiol. 2023 Jul 28;3:943223. doi: 10.3389/fnetp.2023.943223. eCollection 2023. Front Netw Physiol. 2023. PMID: 37577037 Free PMC article. Review.
Cited by
- Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: A meta-analysis and recommendations for future studies.
Beynel L, Appelbaum LG, Luber B, Crowell CA, Hilbig SA, Lim W, Nguyen D, Chrapliwy NA, Davis SW, Cabeza R, Lisanby SH, Deng ZD. Beynel L, et al. Neurosci Biobehav Rev. 2019 Dec;107:47-58. doi: 10.1016/j.neubiorev.2019.08.018. Epub 2019 Aug 29. Neurosci Biobehav Rev. 2019. PMID: 31473301 Free PMC article. Review. - Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex enhances working memory.
Bagherzadeh Y, Khorrami A, Zarrindast MR, Shariat SV, Pantazis D. Bagherzadeh Y, et al. Exp Brain Res. 2016 Jul;234(7):1807-1818. doi: 10.1007/s00221-016-4580-1. Epub 2016 Feb 16. Exp Brain Res. 2016. PMID: 26884132 - Role of Single Low Pulse Intensity of Transcranial Magnetic Stimulation Over the Frontal Cortex for Cognitive Function.
Bashir S, Al-Hussain F, Hamza A, Shareefi GF, Abualait T, Yoo WK. Bashir S, et al. Front Hum Neurosci. 2020 Jul 3;14:205. doi: 10.3389/fnhum.2020.00205. eCollection 2020. Front Hum Neurosci. 2020. PMID: 32719592 Free PMC article. - Attention to memory: orienting attention to sound object representations.
Backer KC, Alain C. Backer KC, et al. Psychol Res. 2014;78(3):439-52. doi: 10.1007/s00426-013-0531-7. Epub 2013 Dec 20. Psychol Res. 2014. PMID: 24352689 Review. - Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing.
Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Mathewson KE, et al. Front Psychol. 2011 May 19;2:99. doi: 10.3389/fpsyg.2011.00099. eCollection 2011. Front Psychol. 2011. PMID: 21779257 Free PMC article.
References
- Berger H. (1929). Über das Elektroenkephalogramm des Menschen. Arch. Psychiatr. Nervenkr. 87, 527–57010.1007/BF01797193 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous