Phenotypic differences between mice deficient in XIAP and SAP, two factors targeted in X-linked lymphoproliferative syndrome (XLP) - PubMed (original) (raw)
Comparative Study
Phenotypic differences between mice deficient in XIAP and SAP, two factors targeted in X-linked lymphoproliferative syndrome (XLP)
Julie M Rumble et al. Cell Immunol. 2009.
Abstract
Mutations in the X-linked inhibitor of apoptosis (XIAP) have recently been identified in patients with the rare genetic disease, X-linked lymphoproliferative syndrome (XLP), which was previously thought to be solely attributable to mutations in a distinct gene, SAP. To further understand the roles of these two factors in the pathogenesis of XLP, we have compared mice deficient in Xiap with known phenotypes of Sap-null mice. We show here that in contrast to Sap-deficient mice, animals lacking Xiap have apparently normal NKT cell development and no apparent defect in humoral responses to T cell-dependent antigens. However, Xiap-deficient cells were more susceptible to death upon infection with the murine herpesvirus MHV-68 and gave rise to more infectious virus. These differences could be rescued by restoration of XIAP. These data provide insight into the differing roles of XIAP and SAP in the pathogenesis of XLP.
Figures
Fig. 1. No detectable interaction between XIAP and SAP
(A) XIAP and FLAG-tagged SAP were coexpressed with the cytoplasmic tail of SLAM-GST or GST alone in HEK293 cells. Glutathione-sepharose beads were added to lysates and bead-associated proteins were separated by SDS-PAGE and immunoblotted for FLAG, GST and XIAP. Additionally, the last panel shows immunoprecipitation using an anti-FLAG monoclonal antibody and IgA beads to assess binding of SLAM and XIAP to SAP. (B) HA-XIAP (both wildtype and a H467A point mutant) and FLAG-SAP were expressed in HEK293 cells in the presence of the tyrosine kinase Lck and either a GST-tagged cytoplasmic tail construct of the 2B4 receptor or GST alone. As in A, GST coprecipitations and immunoblots were performed assessing the ability of wildtype (WT) or D148A/W310A double mutant (MT) XIAP to bind SAP or 2B4. All samples include Lck.
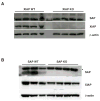
Fig. 2. Murine expression of XIAP and SAP
Thymocytes were harvested from XIAP (A) and SAP (B) WT and KO mice, lysed and immunoblotted for SAP, XIAP and β-actin. Asterisk (*) indicates a non-specific band.
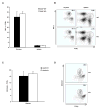
Fig. 3. NKT cells normal in XIAP KO mice
Splenocytes and thymocytes were isolated from three XIAP WT and three XIAP KO mice, and stained with anti-CD24, NK1.1+ and TCR-β+ (A and B), as well as PE-conjugated α-galactosylceramide-loaded CD1d tetramer, specific for NKT cells (C and D). NKT cells are defined as CD24low, NK1.1+, TCR-β+, and tetramer+. A and C show total results of at least 3 individual mice, error bars shown are standard error of the mean. B and D are representative FACS plots of the CD24low subpopulation.
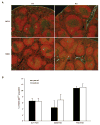
Fig. 4. Normal germinal center formation in XIAP KO mice
(A) XIAP WT and KO mice were injected with either SRBC or saline and spleens were harvested 6 days later. Frozen sections were stained with PNA-FITC and anti-B220 and viewed on an Olympus microscope. (B) Splenocytes were harvested from mice treated in A and stained with antibodies to B220, IgD and Fas. Subsets were also stained with PNA or antibodies to GL7 or CD38 to specifically identify germinal center B cells. Data are representative of at least three individual mice, and error bars shown are standard error of the mean.
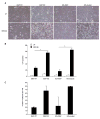
Fig. 5. _Xiap_-null cells are sensitive to virus-induced death
(A) The indicated MEF cell lines were infected with 0.1 pfu/cell MHV-68 and cultured for 72 hours, after which they were visualized with light microscope. (B) Cells were treated as in A, then floating and adherent cells were harvested and PI stained for viability by flow cytometry. Data represent at least three experiments, with error bars illustrating standard error, and significance (<0.001 indicated with an asterisk [*]) was calculated using a one-way ANOVA. (C) Supernatants from cells treated as in A were serially diluted and plated on 3T12 cells for quantitation by plaque assay. Three wells were counted from the 1:32,000 dilution of supernatant in each of two experiments.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article. - Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.
Harnan S, Kearns B, Scope A, Schmitt L, Jankovic D, Hamilton J, Srivastava T, Hill H, Ku CC, Ren S, Rothery C, Bojke L, Sculpher M, Woods B. Harnan S, et al. Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article. - Topical fluoride as a cause of dental fluorosis in children.
Wong MCM, Zhang R, Luo BW, Glenny AM, Worthington HV, Lo ECM. Wong MCM, et al. Cochrane Database Syst Rev. 2024 Jun 20;6(6):CD007693. doi: 10.1002/14651858.CD007693.pub3. Cochrane Database Syst Rev. 2024. PMID: 38899538 Review.
Cited by
- SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions.
Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. Detre C, et al. Semin Immunopathol. 2010 Jun;32(2):157-71. doi: 10.1007/s00281-009-0193-0. Epub 2010 Feb 10. Semin Immunopathol. 2010. PMID: 20146065 Free PMC article. Review. - A mutation in X-linked inhibitor of apoptosis (G466X) leads to memory inflation of Epstein-Barr virus-specific T cells.
Lopez-Granados E, Stacey M, Kienzler AK, Sierro S, Willberg CB, Fox CP, Rigaud S, Long HM, Hislop AD, Rickinson AB, Patel S, Latour S, Klenerman P, Chapel H. Lopez-Granados E, et al. Clin Exp Immunol. 2014 Dec;178(3):470-82. doi: 10.1111/cei.12427. Clin Exp Immunol. 2014. PMID: 25079909 Free PMC article. - Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes.
Marsh RA, Rao K, Satwani P, Lehmberg K, Müller I, Li D, Kim MO, Fischer A, Latour S, Sedlacek P, Barlogis V, Hamamoto K, Kanegane H, Milanovich S, Margolis DA, Dimmock D, Casper J, Douglas DN, Amrolia PJ, Veys P, Kumar AR, Jordan MB, Bleesing JJ, Filipovich AH. Marsh RA, et al. Blood. 2013 Feb 7;121(6):877-83. doi: 10.1182/blood-2012-06-432500. Epub 2012 Nov 6. Blood. 2013. PMID: 23131490 Free PMC article. - CD1d Expression and Invariant NKT Cell Responses in Herpesvirus Infections.
Chung BK, Priatel JJ, Tan R. Chung BK, et al. Front Immunol. 2015 Jun 25;6:312. doi: 10.3389/fimmu.2015.00312. eCollection 2015. Front Immunol. 2015. PMID: 26161082 Free PMC article. Review. - Familial hemophagocytic lymphohistiocytosis: a model for understanding the human machinery of cellular cytotoxicity.
Sieni E, Cetica V, Mastrodicasa E, Pende D, Moretta L, Griffiths G, Aricò M. Sieni E, et al. Cell Mol Life Sci. 2012 Jan;69(1):29-40. doi: 10.1007/s00018-011-0835-y. Epub 2011 Oct 12. Cell Mol Life Sci. 2012. PMID: 21990010 Free PMC article. Review.
References
- Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. - PubMed
- Hoffmann T, Heilmann C, Madsen HO, Vindelov L, Schmiegelow K. Matched unrelated allogeneic bone marrow transplantation for recurrent malignant lymphoma in a patient with X-linked lymphoproliferative disease (XLP) Bone Marrow Transplant. 1998;22:603–604. - PubMed
- Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1:935–940. - PubMed
- Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95:13765–13770. - PMC - PubMed
- Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nature Genetics. 1998;20:129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI065543/AI/NIAID NIH HHS/United States
- R01 AI065543/AI/NIAID NIH HHS/United States
- R01 GM067827-05/GM/NIGMS NIH HHS/United States
- R56 AI065543/AI/NIAID NIH HHS/United States
- CA86867/CA/NCI NIH HHS/United States
- R01 HL087846/HL/NHLBI NIH HHS/United States
- HL087846/HL/NHLBI NIH HHS/United States
- GM067827/GM/NIGMS NIH HHS/United States
- R01 GM067827/GM/NIGMS NIH HHS/United States
- R01 CA086867/CA/NCI NIH HHS/United States
- T32 AI007413/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous