Biology and treatment of eosinophilic esophagitis - PubMed (original) (raw)
Review
Biology and treatment of eosinophilic esophagitis
Marc E Rothenberg. Gastroenterology. 2009 Oct.
Abstract
Eosinophilic esophagitis is a recently recognized but expanding disorder characterized by antigen-driven eosinophil accumulation in the esophagus. Symptoms frequently mimic those of gastroesophageal reflux disease, but the diseases are distinct in their histopathology, gene expression signature, response to therapy, hereditary risk, and association with allergies. The pathogenesis of eosinophilic esophagitis involves environmental and genetic factors, particularly food antigens and expression level of the eosinophil chemoattractant eotaxin-3, respectively. Analyses of gene expression signatures and animal models have indicated the importance of adaptive T-cell immunity that involves interleukin-5 and interleukin-13-induced esophageal epithelial cell responses. Symptoms, dysregulation of esophageal gene expression, and pathology are largely reversible following reduced exposure to specific food antigens as well as anti-inflammatory therapy, but chronic treatment is necessary to prevent relapse. Therefore, eosinophilic esophagitis is a disease with unique features that include chronic esophagitis, atopy, immune sensitization to oral antigens, reversibility, and familial association.
Figures
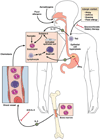
Figure 1. Molecular and cellular mechanisms involved in EE pathogenesis, eotaxin-3-associated eosinophil recruitment and treatments
Aeroallergen, food allergen and skin sensitization have been implicated in EE pathogenesis. Elemental diet, glucocorticoids, and anti-IL-5 treatments improve the microscopic features of EE acting at different levels on the disease pathogenesis. Proton pomp inhibitors (PPI) are important in establishing the diagnosis of EE as inflammation should be present on PPIs therapy. Hyperplasic epithelial cells of the esophagus overexpress eotaxin-3 in response to IL-13. Fibroblasts overexpress periostin and downregulate filaggrin likelyin response to IL-13. Eotaxin-3 and periostin overexpression cooperatively chemoattract CCR3+ cells, a process primed by IL-5 which regulates eosinophil responsiveness to eotaxin-3 and the circulating level of eosinophils. Inheritance of EE disease suggests a genetic predisposition. A SNP in the eotaxin-3 gene has been associated with EE. In addition to eosinophils, mast cells and lymphocytes including B cells accumulate in the esophagus of EE patients and likely contribute to the local inflammatory responses.
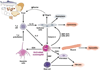
Figure 2. Schematic diagram of the multifunctional effects of eosinophils
Eosinophils are bilobed granulocytes with eosinophilic staining secondary granules. The secondary granules contain four primary cationic proteins designated eosinophil peroxidase (EPO), major basic protein (MBP), eosinophil cationic protein (ECP), and eosinophil derived neurotoxin (EDN). EDN is a ligand for Toll like receptor (TLR)2 and induces the Th2 polarizing ability of dendritic cells. All four proteins are cytotoxic molecules; in addition, ECP and EDN are ribonucleases. Eosinophils respond to diverse stimuli including non-specific tissue injury, infections, allergens and tumors. In addition to releasing their preformed cationic proteins, eosinophils also release a variety of cytokines, chemokines, lipid mediators, and neuro-modulators. Eosinophils directly communicate with T cells and mast cells in a bidirectional manner. Eosinophils are capable of catapulting their mitochrondrial DNA which can serve as extracellular traps for bacteria. Eosinophil derived TGF-β induces fibrosis.
Figure 3. Molecular regulation of the Th2 inflammatory response in EE
Food antigen triggered T helper type 2 (Th2) cells release IL-5 and IL-13 which target eosinophils and esophageal epithelial cells, respectively. IL-13 triggered responses include marked production of the eosinophil chemoattactrant and activating factor eotaxin-3 by epithelial cells as well as down regulation of filaggrin expression. Activated eosinophils release MBP and EDN which target mast cells and dendritic cells, respectively. Eosinophil derived TGF-β and MBP target fibroblasts, epithelial cells and smooth muscle cells and contribute to cellular hyperplasia, fibrosis and dysmotility. In addition, mast cell activation contributes to fibrosis. Perhaps reduced esophageal barrier function (mediated by decreased filaggrin) perpetuates the process by promoting local food antigen uptake. Genetic variants in regulatory molecules involved in these steps may contribute to EE disease risk.
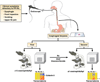
Figure 4. Molecular and histological diagnosis of EE
Patients with PPI refractory upper gastrointestinal symptoms undergo endoscopic biopsy. Tissue is analyzed microscopically and a minimal level of 15 peak eosinophils per hpf is required for diagnosis. In addition, molecular profiling reveals dysregulated expression of 1% of the human genome including eotaxin-3 overexpression that readily distinguishes biopsies from EE, reflux esophagitis (RE) and normal individuals (NL). Following treatment (Rx) with dietary modification and/or glucocorticoids, endoscopic analysis reveals complete resolution of esophageal eosinophilia and large normalization of the EE transcriptome. Residual abnormal gene expression (*) differentiates treated EE from NL and esophagitis RE patients.
Similar articles
- Eosinophilic esophagitis: pathogenesis, genetics, and therapy.
Blanchard C, Wang N, Rothenberg ME. Blanchard C, et al. J Allergy Clin Immunol. 2006 Nov;118(5):1054-9. doi: 10.1016/j.jaci.2006.07.038. Epub 2006 Sep 18. J Allergy Clin Immunol. 2006. PMID: 17088129 Review. - Mechanism of eosinophilic esophagitis.
Mishra A. Mishra A. Immunol Allergy Clin North Am. 2009 Feb;29(1):29-40, viii. doi: 10.1016/j.iac.2008.09.010. Immunol Allergy Clin North Am. 2009. PMID: 19141339 Free PMC article. Review. - Eosinophilic esophagitis: an update.
Ferguson DD, Foxx-Orenstein AE. Ferguson DD, et al. Dis Esophagus. 2007;20(1):2-8. doi: 10.1111/j.1442-2050.2007.00649.x. Dis Esophagus. 2007. PMID: 17227302 Review. - Primary eosinophilic esophagitis.
Munitiz V, Martinez de Haro LF, Ortiz A, Pons JA, Bermejo J, Serrano A, Molina J, Parrilla P. Munitiz V, et al. Dis Esophagus. 2003;16(2):165-8. doi: 10.1046/j.1442-2050.2003.00319.x. Dis Esophagus. 2003. PMID: 12823222 Review. - Eosinophilic esophagitis: management and pharmacotherapy.
De Angelis P, Morino G, Pane A, Torroni F, Francalanci P, Sabbi T, Foschia F, Caldaro T, di Abriola GF, Dall'Oglio L. De Angelis P, et al. Expert Opin Pharmacother. 2008 Apr;9(5):731-40. doi: 10.1517/14656566.9.5.731. Expert Opin Pharmacother. 2008. PMID: 18345951 Review.
Cited by
- Eosinophilic Esophagitis and Inflammatory Bowel Disease: What Are the Differences?
Melhem H, Niess JH. Melhem H, et al. Int J Mol Sci. 2024 Aug 5;25(15):8534. doi: 10.3390/ijms25158534. Int J Mol Sci. 2024. PMID: 39126102 Free PMC article. Review. - An efficient computational framework for gastrointestinal disorder prediction using attention-based transfer learning.
Zhou J, Song W, Liu Y, Yuan X. Zhou J, et al. PeerJ Comput Sci. 2024 May 28;10:e2059. doi: 10.7717/peerj-cs.2059. eCollection 2024. PeerJ Comput Sci. 2024. PMID: 38855223 Free PMC article. - Causality of Helicobacter pylori infection on eosinophilic esophagitis and potential pathogenesis: a Mendelian randomization study.
Zhu Z, Yang Y, Han X, Peng L, Zhu H. Zhu Z, et al. Front Immunol. 2024 May 8;15:1365604. doi: 10.3389/fimmu.2024.1365604. eCollection 2024. Front Immunol. 2024. PMID: 38779684 Free PMC article. - Causal relationship between eosinophilic esophagitis and inflammatory bowel disease: a bidirectional two-sample Mendelian randomization study.
Ji R, Zhi Y. Ji R, et al. Front Immunol. 2024 Apr 24;15:1374107. doi: 10.3389/fimmu.2024.1374107. eCollection 2024. Front Immunol. 2024. PMID: 38720886 Free PMC article. - Fibrous Remodeling in Eosinophilic Esophagitis: Clinical Facts and Pathophysiological Uncertainties.
Arias-González L, Rodríguez-Alcolado L, Laserna-Mendieta EJ, Navarro P, Lucendo AJ, Grueso-Navarro E. Arias-González L, et al. Int J Mol Sci. 2024 Jan 11;25(2):927. doi: 10.3390/ijms25020927. Int J Mol Sci. 2024. PMID: 38256003 Free PMC article. Review.
References
- Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113:11–28. - PubMed
- Balatsinou C, Milano A, Caldarella MP, Laterza F, Pierdomenico SD, Cuccurullo F, Neri M. Eosinophilic esophagitis is a component of the anticonvulsant hypersensitivity syndrome: Description of two cases. Dig Liver Dis. 2007 - PubMed
- Noble C, Francis L, Withers GW, Ee LC, Lewindon PJ. Audit of eosinophilic oesophagitis in children post-liver transplant. Pediatr Transplant. 2008 - PubMed
- Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI080581/AI/NIAID NIH HHS/United States
- R01 AI45898/AI/NIAID NIH HHS/United States
- R01 AI05780/AI/NIAID NIH HHS/United States
- U19 AI070235/AI/NIAID NIH HHS/United States
- R01 DK076893/DK/NIDDK NIH HHS/United States
- R01 AI057803/AI/NIAID NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- R37 AI045898/AI/NIAID NIH HHS/United States
- R01 DK067255/DK/NIDDK NIH HHS/United States
- R01 AI045898/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical