De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot - PubMed (original) (raw)
doi: 10.1038/ng.415. Epub 2009 Jul 13.
Alexandre C Pereira, Jennifer C Lin, Steven R DePalma, Samuel J Israel, Sonia M Mesquita, Emel Ergul, Jessie H Conta, Joshua M Korn, Steven A McCarroll, Joshua M Gorham, Stacey Gabriel, David M Altshuler, Maria de Lourdes Quintanilla-Dieck, Maria Alexandra Artunduaga, Roland D Eavey, Robert M Plenge, Nancy A Shadick, Michael E Weinblatt, Philip L De Jager, David A Hafler, Roger E Breitbart, Jonathan G Seidman, Christine E Seidman
Affiliations
- PMID: 19597493
- PMCID: PMC2747103
- DOI: 10.1038/ng.415
De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot
Steven C Greenway et al. Nat Genet. 2009 Aug.
Abstract
Tetralogy of Fallot (TOF), the most common severe congenital heart malformation, occurs sporadically, without other anomaly, and from unknown cause in 70% of cases. Through a genome-wide survey of 114 subjects with TOF and their unaffected parents, we identified 11 de novo copy number variants (CNVs) that were absent or extremely rare (<0.1%) in 2,265 controls. We then examined a second, independent TOF cohort (n = 398) for additional CNVs at these loci. We identified CNVs at chromosome 1q21.1 in 1% (5/512, P = 0.0002, OR = 22.3) of nonsyndromic sporadic TOF cases. We also identified recurrent CNVs at 3p25.1, 7p21.3 and 22q11.2. CNVs in a single subject with TOF occurred at six loci, two that encode known (NOTCH1, JAG1) disease-associated genes. Our findings predict that at least 10% (4.5-15.5%, 95% confidence interval) of sporadic nonsyndromic TOF cases result from de novo CNVs and suggest that mutations within these loci might be etiologic in other cases of TOF.
Figures
Figure 1
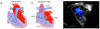
Anatomy and pathophysiology of tetralogy of Fallot (TOF). Normal heart structure (a) promotes unidirectional flow of deoxygenated blood (blue) into the lungs and oxygenated blood (red) into the aorta. In TOF (b) pulmonary stenosis and narrowing of the right ventricular outflow tract (RVOT) impedes the flow of deoxygenated blood into the lungs, and both the ventricular septal defect (VSD) and overriding aorta (*) promote the flow of deoxygenated blood into the systemic circulation, to produce cyanosis (sometimes referred to as “blue baby” syndrome). Right ventricular hypertrophy (RVH) is also present. (c) A Doppler echocardiogram shows mixing of deoxygenated blood from the right ventricle (RV) and oxygenated blood from the left ventricle (LV) as blood is pumped out the overriding aorta (Ao) in a patient with TOF. RA, right atrium; LA, left atrium. Images from Multimedia Library of Congenital Heart Disease, Children’s Hospital, Boston, MA, editor Robert Geggel, MD,
www.childrenshospital.org/mml/cvp
, with permission.
Figure 2
CNVs associated with TOF. (a) Duplications in four TOF patients (749, 201.670, 200.430, 200.250) and a deletion (patient 3701) overlap a 875,266 bp region at 1q21 (chr1:144,965,244-145,840,510) which encompasses six known genes that are expressed in the human right ventricular outflow tract. Previously described duplications at this locus are associated with mental retardation (MR), TOF, macrocephaly (MaC) and other congenital phenotypes. Studies have identified multiple patients (number of patients in parentheses, only the minimal overlapping region between patients is shown) that carry deletions at this locus with congenital heart disease other than TOF (CHD), MR, schizophrenia (SCZ), microcephaly (MiC) or that carry deletions with CHD and MR. (b) Plot showing normalized probe intensity measurements across 1q21 in the four TOF patients carrying a deletion (turquoise), the one TOF patient with a duplication (green) and 273 CN-neutral controls (gray lines and summarized as mean (black) ± 2*median absolute deviation (dark blue lines). The vertical dotted lines indicate the boundaries of the 875,266 bp overlapping region. The absence of circles on the colored lines indicates an absence of probes due to segmental duplications and there is a known CNP downstream of our region of interest. (c) A 12,380,330 bp duplication on chromosome 3 was found in a single TOF patient (756) and an inherited duplication was found within this interval in patient 419 which narrows the interval to a region (chr3:12,605,755-12,781,130) affecting RAF1. White bars indicate deletion, black bars indicate duplication, and red bars indicate the region of overlap between TOF CNVs. Chromosome position is indicated in Mb by the blue bar. All coordinates are based on build 36.1 of the human reference genome.
Figure 2
CNVs associated with TOF. (a) Duplications in four TOF patients (749, 201.670, 200.430, 200.250) and a deletion (patient 3701) overlap a 875,266 bp region at 1q21 (chr1:144,965,244-145,840,510) which encompasses six known genes that are expressed in the human right ventricular outflow tract. Previously described duplications at this locus are associated with mental retardation (MR), TOF, macrocephaly (MaC) and other congenital phenotypes. Studies have identified multiple patients (number of patients in parentheses, only the minimal overlapping region between patients is shown) that carry deletions at this locus with congenital heart disease other than TOF (CHD), MR, schizophrenia (SCZ), microcephaly (MiC) or that carry deletions with CHD and MR. (b) Plot showing normalized probe intensity measurements across 1q21 in the four TOF patients carrying a deletion (turquoise), the one TOF patient with a duplication (green) and 273 CN-neutral controls (gray lines and summarized as mean (black) ± 2*median absolute deviation (dark blue lines). The vertical dotted lines indicate the boundaries of the 875,266 bp overlapping region. The absence of circles on the colored lines indicates an absence of probes due to segmental duplications and there is a known CNP downstream of our region of interest. (c) A 12,380,330 bp duplication on chromosome 3 was found in a single TOF patient (756) and an inherited duplication was found within this interval in patient 419 which narrows the interval to a region (chr3:12,605,755-12,781,130) affecting RAF1. White bars indicate deletion, black bars indicate duplication, and red bars indicate the region of overlap between TOF CNVs. Chromosome position is indicated in Mb by the blue bar. All coordinates are based on build 36.1 of the human reference genome.
Figure 2
CNVs associated with TOF. (a) Duplications in four TOF patients (749, 201.670, 200.430, 200.250) and a deletion (patient 3701) overlap a 875,266 bp region at 1q21 (chr1:144,965,244-145,840,510) which encompasses six known genes that are expressed in the human right ventricular outflow tract. Previously described duplications at this locus are associated with mental retardation (MR), TOF, macrocephaly (MaC) and other congenital phenotypes. Studies have identified multiple patients (number of patients in parentheses, only the minimal overlapping region between patients is shown) that carry deletions at this locus with congenital heart disease other than TOF (CHD), MR, schizophrenia (SCZ), microcephaly (MiC) or that carry deletions with CHD and MR. (b) Plot showing normalized probe intensity measurements across 1q21 in the four TOF patients carrying a deletion (turquoise), the one TOF patient with a duplication (green) and 273 CN-neutral controls (gray lines and summarized as mean (black) ± 2*median absolute deviation (dark blue lines). The vertical dotted lines indicate the boundaries of the 875,266 bp overlapping region. The absence of circles on the colored lines indicates an absence of probes due to segmental duplications and there is a known CNP downstream of our region of interest. (c) A 12,380,330 bp duplication on chromosome 3 was found in a single TOF patient (756) and an inherited duplication was found within this interval in patient 419 which narrows the interval to a region (chr3:12,605,755-12,781,130) affecting RAF1. White bars indicate deletion, black bars indicate duplication, and red bars indicate the region of overlap between TOF CNVs. Chromosome position is indicated in Mb by the blue bar. All coordinates are based on build 36.1 of the human reference genome.
Similar articles
- Rare copy number variations in adults with tetralogy of Fallot implicate novel risk gene pathways.
Silversides CK, Lionel AC, Costain G, Merico D, Migita O, Liu B, Yuen T, Rickaby J, Thiruvahindrapuram B, Marshall CR, Scherer SW, Bassett AS. Silversides CK, et al. PLoS Genet. 2012;8(8):e1002843. doi: 10.1371/journal.pgen.1002843. Epub 2012 Aug 9. PLoS Genet. 2012. PMID: 22912587 Free PMC article. - Identification of novel rare copy number variants associated with sporadic tetralogy of Fallot and clinical implications.
He GW, Maslen CL, Chen HX, Hou HT, Bai XY, Wang XL, Liu XC, Lu WL, Chen XX, Chen WD, Xing QS, Wu Q, Wang J, Yang Q. He GW, et al. Clin Genet. 2022 Nov;102(5):391-403. doi: 10.1111/cge.14201. Epub 2022 Aug 2. Clin Genet. 2022. PMID: 35882632 - Ultra high-resolution gene centric genomic structural analysis of a non-syndromic congenital heart defect, Tetralogy of Fallot.
Bittel DC, Zhou XG, Kibiryeva N, Fiedler S, O'Brien JE Jr, Marshall J, Yu S, Liu HY. Bittel DC, et al. PLoS One. 2014 Jan 31;9(1):e87472. doi: 10.1371/journal.pone.0087472. eCollection 2014. PLoS One. 2014. PMID: 24498113 Free PMC article. Clinical Trial. - Human Genetics of Tetralogy of Fallot and Double-Outlet Right Ventricle.
Dorn C, Perrot A, Grunert M, Rickert-Sperling S. Dorn C, et al. Adv Exp Med Biol. 2024;1441:629-644. doi: 10.1007/978-3-031-44087-8_36. Adv Exp Med Biol. 2024. PMID: 38884738 Review. - De novo 9q gain in an infant with tetralogy of Fallot with absent pulmonary valve: Patient report and review of congenital heart disease in 9q duplication syndrome.
Amarillo IE, O'Connor S, Lee CK, Willing M, Wambach JA. Amarillo IE, et al. Am J Med Genet A. 2015 Dec;167A(12):2966-74. doi: 10.1002/ajmg.a.37296. Epub 2015 Aug 19. Am J Med Genet A. 2015. PMID: 26768185 Free PMC article. Review.
Cited by
- Genome-Wide Association Study of Down Syndrome-Associated Atrioventricular Septal Defects.
Ramachandran D, Zeng Z, Locke AE, Mulle JG, Bean LJ, Rosser TC, Dooley KJ, Cua CL, Capone GT, Reeves RH, Maslen CL, Cutler DJ, Feingold E, Sherman SL, Zwick ME. Ramachandran D, et al. G3 (Bethesda). 2015 Jul 20;5(10):1961-71. doi: 10.1534/g3.115.019943. G3 (Bethesda). 2015. PMID: 26194203 Free PMC article. - 1q21.1 microduplication in a patient with mental impairment and congenital heart defect.
Sun G, Tan Z, Fan L, Wang J, Yang Y, Zhang W. Sun G, et al. Mol Med Rep. 2015 Oct;12(4):5655-8. doi: 10.3892/mmr.2015.4166. Epub 2015 Jul 31. Mol Med Rep. 2015. PMID: 26238956 Free PMC article. - Cytogenomic Aberrations in Congenital Cardiovascular Malformations.
Azamian M, Lalani SR. Azamian M, et al. Mol Syndromol. 2016 May;7(2):51-61. doi: 10.1159/000445788. Epub 2016 Apr 26. Mol Syndromol. 2016. PMID: 27385961 Free PMC article. Review. - Copy number variation analysis in bicuspid aortic valve-related aortopathy identifies TBX20 as a contributing gene.
Luyckx I, Kumar AA, Reyniers E, Dekeyser E, Vanderstraeten K, Vandeweyer G, Wünnemann F, Preuss C, Mazzella JM, Goudot G, Messas E, Albuisson J, Jeunemaitre X, Eriksson P, Mohamed SA, Kempers M, Salemink S, Duijnhouwer A, Andelfinger G, Dietz HC, Verstraeten A, Van Laer L, Loeys BL; MIBAVA Leducq Consortium. Luyckx I, et al. Eur J Hum Genet. 2019 Jul;27(7):1033-1043. doi: 10.1038/s41431-019-0364-y. Epub 2019 Feb 28. Eur J Hum Genet. 2019. PMID: 30820038 Free PMC article. Clinical Trial. - Genetic counseling in the adult with congenital heart disease: what is the role?
Burchill L, Greenway S, Silversides CK, Mital S. Burchill L, et al. Curr Cardiol Rep. 2011 Aug;13(4):347-55. doi: 10.1007/s11886-011-0188-z. Curr Cardiol Rep. 2011. PMID: 21537992
References
- Ferencz C, et al. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol. 1985;121:31–36. - PubMed
- Schott JJ, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. - PubMed
- Basson CT, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. - PubMed
- Eldadah ZA, et al. Familial Tetralogy of Fallot caused by mutation in the jagged1 gene. Hum Mol Genet. 2001;10:163–169. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources