Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala - PubMed (original) (raw)
Meta-Analysis
Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala
Jennifer L Robinson et al. Hum Brain Mapp. 2010 Feb.
Abstract
Functional neuroimaging has evolved into an indispensable tool for noninvasively investigating brain function. A recent development of such methodology is the creation of connectivity models for brain regions and related networks, efforts that have been inhibited by notable limitations. We present a new method for ascertaining functional connectivity of specific brain structures using metaanalytic connectivity modeling (MACM), along with validation of our method using a nonhuman primate database. Drawing from decades of neuroimaging research and spanning multiple behavioral domains, the method overcomes many weaknesses of conventional connectivity analyses and provides a simple, automated alternative to developing accurate and robust models of anatomically-defined human functional connectivity. Applying MACM to the amygdala, a small structure of the brain with a complex network of connections, we found high coherence with anatomical studies in nonhuman primates as well as human-based theoretical models of emotive-cognitive integration, providing evidence for this novel method's utility.
(c) 2009 Wiley-Liss, Inc.
Figures
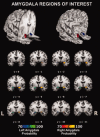
Figure 1
Anatomical 3D‐renderings of the amygdala ROIs used for the meta‐analysis. The right amygdala is represented in red and the left amygdala is represented in blue. Figure created using Mango (
).
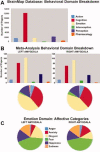
Figure 2
Papers reporting activation within the left and right amygdala ROIs were drawn from the BrainMap Database for subsequent metaanalysis. The graphs below demonstrate the number of papers coded in each behavioral domain in the entire BrainMap database (a), as well as the behavioral domain breakdown of the papers included in the amygdala‐specific meta‐analysis (b). From the emotion domain, which contained the highest percentage of papers, a variety of affective states were represented (c).
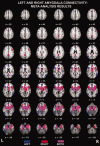
Figure 3
Coactivation patterns obtained from meta‐analysis with the left (blue) and right (red) amygdala. Regions of the brain that coactivated with both left and right amygdala are indicated in purple. Maps thresholded at P < 0.001. Figure created using Mango (
).
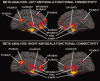
Figure 4
The 3D renderings of coactivation patterns for the left (top panel) and right (bottom panel) amygdala. AC = anterior cingulate; BA = Brodmann area; MFG = medial frontal gyrus; PC = posterior cingulate cortex; PHIPP = parahippocampal gyrus; SFG = superior frontal gyrus; THAL = thalamus.
Figure 5
Images were constructed using the CoCoMac‐Paxinos3D Viewer. Panel A shows all 151 slices of the atlas in stereotaxic space. Within each of the panels B–F, the brain region is displayed in this space (left side of each panel) in addition to a magnified image of the connectivity results. Please see Table IV for a list of all abbreviations. B = Anterior amygdaloid area, C = Amygdalopiriform transition area, D = Basolateral amygdaloid nucleus, E = Basomedial amygdaloid nucleus, F = Central medial amygdaloid nucleus, medial division. Blue arrows indicate evidence for no anatomical connectivity, gray represents anatomical connectivity of unknown density, yellow is weak anatomical connectivity, and red indicates strong anatomical connections.
Similar articles
- Exploring the brain network: a review on resting-state fMRI functional connectivity.
van den Heuvel MP, Hulshoff Pol HE. van den Heuvel MP, et al. Eur Neuropsychopharmacol. 2010 Aug;20(8):519-34. doi: 10.1016/j.euroneuro.2010.03.008. Epub 2010 May 14. Eur Neuropsychopharmacol. 2010. PMID: 20471808 Review. - Modulating functional connectivity between medial frontopolar cortex and amygdala by inhibitory and excitatory transcranial magnetic stimulation.
Riedel P, Heil M, Bender S, Dippel G, Korb FM, Smolka MN, Marxen M. Riedel P, et al. Hum Brain Mapp. 2019 Oct 15;40(15):4301-4315. doi: 10.1002/hbm.24703. Epub 2019 Jul 3. Hum Brain Mapp. 2019. PMID: 31268615 Free PMC article. - Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis.
Cauda F, Cavanna AE, D'agata F, Sacco K, Duca S, Geminiani GC. Cauda F, et al. J Cogn Neurosci. 2011 Oct;23(10):2864-77. doi: 10.1162/jocn.2011.21624. Epub 2011 Jan 25. J Cogn Neurosci. 2011. PMID: 21265603 - Mapping effective connectivity of human amygdala subdivisions with intracranial stimulation.
Sawada M, Adolphs R, Dlouhy BJ, Jenison RL, Rhone AE, Kovach CK, Greenlee JDW, Howard Iii MA, Oya H. Sawada M, et al. Nat Commun. 2022 Aug 20;13(1):4909. doi: 10.1038/s41467-022-32644-y. Nat Commun. 2022. PMID: 35987994 Free PMC article. - Biophysical and neural basis of resting state functional connectivity: Evidence from non-human primates.
Chen LM, Yang PF, Wang F, Mishra A, Shi Z, Wu R, Wu TL, Wilson GH 3rd, Ding Z, Gore JC. Chen LM, et al. Magn Reson Imaging. 2017 Jun;39:71-81. doi: 10.1016/j.mri.2017.01.020. Epub 2017 Feb 2. Magn Reson Imaging. 2017. PMID: 28161319 Free PMC article. Review.
Cited by
- Amygdala self-neuromodulation capacity as a window for process-related network recruitment.
Gurevitch G, Lubianiker N, Markovits T, Or-Borichev A, Sharon H, Fine NB, Fruchtman-Steinbok T, Keynan JN, Shahar M, Friedman A, Singer N, Hendler T. Gurevitch G, et al. Philos Trans R Soc Lond B Biol Sci. 2024 Dec 2;379(1915):20240186. doi: 10.1098/rstb.2024.0186. Epub 2024 Oct 21. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39428877 Free PMC article. - The frontal association area: exercise-induced brain plasticity in children and adolescents and implications for cognitive intervention practice.
Zhang Z, Shi P, Zhang K, Li C, Feng X. Zhang Z, et al. Front Hum Neurosci. 2024 Sep 5;18:1418803. doi: 10.3389/fnhum.2024.1418803. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39301538 Free PMC article. - The neural network of sensory attenuation: A neuroimaging meta-analysis.
Gu J, Buidze T, Zhao K, Gläscher J, Fu X. Gu J, et al. Psychon Bull Rev. 2024 Jul 1. doi: 10.3758/s13423-024-02532-1. Online ahead of print. Psychon Bull Rev. 2024. PMID: 38954157 Review. - Contributions of the left and right thalami to language: A meta-analytic approach.
Bulut T, Hagoort P. Bulut T, et al. Brain Struct Funct. 2024 Apr 16. doi: 10.1007/s00429-024-02795-3. Online ahead of print. Brain Struct Funct. 2024. PMID: 38625556 - Functional decoding and meta-analytic connectivity modeling in thyroid-associated ophthalmopathy.
Duan Q, Wang Z, Cheung W, Liu J, Zhang H, Qiao W, Zhang Q. Duan Q, et al. Heliyon. 2023 Dec 15;10(1):e23749. doi: 10.1016/j.heliyon.2023.e23749. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38226223 Free PMC article.
References
- Abercrombie HC,Schaefer SM,Larson CL,Oakes TR,Lindgren KA,Holden JE,Perlman SB,Turski PA,Krahn DD,Benca RM,Davidson RJ ( 1998): Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport 9: 3301–3307. - PubMed
- Adolphs R,Gosselin F,Buchanan TW,Tranel D,Schyns P,Damasio AR ( 2005): A mechanism for impaired fear recognition after amygdala damage. Nature 433: 68–72. - PubMed
- Aggleton JP,Burton MJ,Passingham RE ( 1980): Cortical and subcortical afferents to the amygdala of the rhesus monkey (macaca mulatta). Brain Res 190: 347–368. - PubMed
- Amaral DG,Price JL ( 1984): Amygdalo‐cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230: 465–496. - PubMed
- Anderson AK,Phelps EA ( 2000): Expression without recognition: Contributions of the human amygdala to emotional communication. Psychol Sci 11: 106–111. - PubMed
Publication types
MeSH terms
Grants and funding
- R56 MH074457/MH/NIMH NIH HHS/United States
- R01 MH074457/MH/NIMH NIH HHS/United States
- R01 AA012207/AA/NIAAA NIH HHS/United States
- R01-MH074457-01A1/MH/NIMH NIH HHS/United States
- R01 MH074457-03/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources