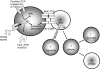mTOR: taking cues from the immune microenvironment - PubMed (original) (raw)
Review
mTOR: taking cues from the immune microenvironment
Greg M Delgoffe et al. Immunology. 2009 Aug.
Abstract
The ultimate outcome of T cell receptor recognition is determined by the context in which the antigen is encountered. In this fashion both antigen-presenting cells and T cells must integrate multiple environmental cues in the form of pathogen-associated molecular patterns, cytokines and accessory molecule signals. The mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase that plays a central role in integrating environmental signals critical to regulating metabolism and cell survival. In this paper we review the data demonstrating that mTOR integrates signals from the immune microenvironment and therefore facilitates the generation of the adaptive immune response. Specifically, we review the role of mTOR in promoting dendritic cell activation and maturation, in regulating full T cell activation versus anergy, and influencing the induction of regulatory T cells.
Figures
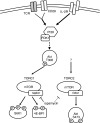
Figure 1
Schematic of mammalian target of rapamycin (mTOR) signalling in T cells. Environmental cues including costimulatory molecule engagement (CD28), growth factors [for example, interleukin-2 (IL-2)], amino acids, and insulin stimulate the activity of the phosphatidylinositol 3-kinase (PI3K) signalling cascade, promoting the partial activation of Akt on threonine 308, upstream of mTOR. The mTOR signalling proceeds via two complexes, TORC1 (characterized by mTOR and raptor) and TORC2 (characterized by mTOR and rictor). While TORC1 is activated in part by the inhibition of TSC2, a biochemical inhibitor of TORC1, the precise upstream events leading to TORC2 activation remain unclear. TORC1 phosphorylates (among many substrates) the ribosomal S6K1 and the translational repressor 4E-BP1. TORC2 phosphorylates Akt on serine 473, locking it into a fully activated conformation. Rapamycin interferes with TORC1 signalling by interfering with mTOR–raptor interactions. Rapamycin can also inhibit TORC2 signalling, although the mechanism is not entirely clear.
Figure 2
Environmental signals (Danger) leading to the activation of mammalian target of rapamycin (mTOR) can induce the activation and maturation of immature DCs. This activation leads to the up-regulation of B7 molecules and the production of type I interferons. In mature myeloid dendritic cells (mDCs) and monocyte-derived lineages, persistent Toll-like receptor (TLR) stimulation may stimulate mTOR to induce a negative feedback loop, leading to the inhibition of pro-inflammatory cytokine secretion. Naïve T cells receiving stimulation in the context of mTOR activation differentiate into effector cells, while those receiving T cell receptor stimulation with decreased mTOR activation (or rapamycin treatment) default into regulatory T cells. Furthermore, differentiated effector cells receiving stimulation with full mTOR activation become fully activated, while those receiving suboptimal mTOR activation are rendered anergic.
Similar articles
- A role for mammalian target of rapamycin in regulating T cell activation versus anergy.
Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. Zheng Y, et al. J Immunol. 2007 Feb 15;178(4):2163-70. doi: 10.4049/jimmunol.178.4.2163. J Immunol. 2007. PMID: 17277121 - Regulation of immune responses by mTOR.
Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Powell JD, et al. Annu Rev Immunol. 2012;30:39-68. doi: 10.1146/annurev-immunol-020711-075024. Epub 2011 Nov 29. Annu Rev Immunol. 2012. PMID: 22136167 Free PMC article. Review. - The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism.
Powell JD, Delgoffe GM. Powell JD, et al. Immunity. 2010 Sep 24;33(3):301-11. doi: 10.1016/j.immuni.2010.09.002. Immunity. 2010. PMID: 20870173 Free PMC article. Review. - mTOR-Mediated Regulation of Dendritic Cell Differentiation and Function.
Sukhbaatar N, Hengstschläger M, Weichhart T. Sukhbaatar N, et al. Trends Immunol. 2016 Nov;37(11):778-789. doi: 10.1016/j.it.2016.08.009. Epub 2016 Sep 7. Trends Immunol. 2016. PMID: 27614799 Free PMC article. Review. - Immunoregulatory functions of mTOR inhibition.
Thomson AW, Turnquist HR, Raimondi G. Thomson AW, et al. Nat Rev Immunol. 2009 May;9(5):324-37. doi: 10.1038/nri2546. Nat Rev Immunol. 2009. PMID: 19390566 Free PMC article. Review.
Cited by
- T-Cell Immune Imbalance in Rheumatoid Arthritis Is Associated with Alterations in NK Cells and NK-Like T Cells Expressing CD38.
Wang H, Fang K, Yan W, Chang X. Wang H, et al. J Innate Immun. 2022;14(2):148-166. doi: 10.1159/000516642. Epub 2021 Aug 24. J Innate Immun. 2022. PMID: 34428762 Free PMC article. - Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis.
Fearon U, Canavan M, Biniecka M, Veale DJ. Fearon U, et al. Nat Rev Rheumatol. 2016 Jul;12(7):385-97. doi: 10.1038/nrrheum.2016.69. Epub 2016 May 26. Nat Rev Rheumatol. 2016. PMID: 27225300 Review. - Hypoxia-inducible factor 1: A link between metabolism and T cell differentiation and a potential therapeutic target.
Pan F, Barbi J, Pardoll DM. Pan F, et al. Oncoimmunology. 2012 Jul 1;1(4):510-515. doi: 10.4161/onci.19457. Oncoimmunology. 2012. PMID: 22754770 Free PMC article. - Interface of signal transduction inhibition and immunotherapy in melanoma.
Shada AL, Molhoek KR, Slingluff CL Jr. Shada AL, et al. Cancer J. 2010 Jul-Aug;16(4):360-6. doi: 10.1097/PPO.0b013e3181eb3393. Cancer J. 2010. PMID: 20693848 Free PMC article. Review. - Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis.
Fearon U, Hanlon MM, Wade SM, Fletcher JM. Fearon U, et al. Clin Exp Immunol. 2019 Aug;197(2):170-180. doi: 10.1111/cei.13228. Epub 2018 Nov 11. Clin Exp Immunol. 2019. PMID: 30357805 Free PMC article. Review.
References
- Bretscher P, Cohn M. A theory of self–nonself discrimination. Science. 1970;169:1042–9. - PubMed
- Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. - PubMed
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. - PubMed
- Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. - PubMed
- Medzhitov R, Janeway CA., Jr How does the immune system distinguish self from nonself? Semin Immunol. 2000;12:185–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous