Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer - PubMed (original) (raw)
Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer
Aaron S Mansfield et al. BMC Cancer. 2009.
Abstract
Background: There is evidence that the immune systems of patients with breast cancer are dysfunctional. Regulatory T cells (Tregs), and IDO, an immunosuppressive enzyme, are associated with more advanced disease in some cancers and may promote immunologic tolerance to tumors. Our aim was to assess whether expression of Foxp3, a marker of Tregs, and IDO were linked with nodal metastasis in breast cancer patients. Inhibitors of IDO are available and could potentially demonstrate utility in breast cancer if IDO drives progression of disease.
Methods: Sentinel lymph nodes (SLN) of 47 breast cancer patients with varying degrees of nodal disease and 10 controls were evaluated for expression of Foxp3 and IDO using immunohistochemistry. Positively stained cells were quantified and their distribution within the SLN noted.
Results: The proportion of Foxp3+ cells was higher in SLN of cancer patients than controls (19% v. 10%, p < 0.001). Specifically, there were more Foxp3+ cells in SLN with metastasis than tumor-free SLN (20% v. 14%, p = 0.02). The proportion IDO+ cell in SLN of cancer patients was not statistically different than controls (4.0% v. 1.6%, p = 0.08). In order to demonstrate the combined immunosuppressive effect of Foxp3 and IDO, we categorized each SLN as positive or negative for Foxp3 and IDO. The Foxp3+/IDO+ group almost exclusively consisted of cancer patients with node positive disease.
Conclusion: In conclusion, our study shows that Foxp3+ cells are associated with more advanced disease in breast cancer, a finding that is proving to be true in many other cancers. As IDO has been found to promote differentiation of Tregs, IDO may become a suitable target to abrogate the development of T-cell tolerance and to promote an effective immune response to breast cancer. Our results about the combined expression of IDO and Foxp3 in metastastic SLN support this assumption.
Figures
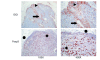
Figure 1
Foxp3 and IDO expression in the sentinel lymph nodes (SLN). This figure illustrates the expression of IDO (top row) and Foxp3 (bottom row) in a SLN with a 7 mm breast cancer metastasis at 100× (first column), and 400× (second column) magnification. Foxp3+ cells are located primarily in the paracortical regions, whereas IDO+ cells infiltrate perisinusoidal areas. The pericapsular fat is marked by the circle, the paracortex by the diamond, the marginal sinus by the arrowhead, and the medullary sinus by the diamond.
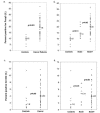
Figure 2
Foxp3 and IDO expression in the sentinel lymph nodes of breast cancer patients and controls. The expression of Foxp3 is shown as the median percentage of positive cells in the sentinel lymph nodes of breast cancer patients and controls (a), and of node-positive, node-negative breast cancer patients and controls (b). The expression of IDO is shown as the median percentage of positive cells in the sentinel lymph nodes of breast cancer patients and controls (c), and of node-positive, node-negative breast cancer patients and controls (d).
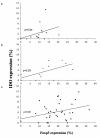
Figure 3
Correlation of the infiltration of Foxp3+ and IDO+ cells. The correlation of Foxp3 and IDO expression of controls (a), node-negative (b), and node-positive (c) patients is graphed above. There were significant correlations in the expression of both markers between the sentinel lymph nodes of the controls (p = 0.04) and those of patients with tumor-free sentinel lymph nodes (p = 0.003).
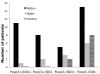
Figure 4
Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. In order to demonstrate the combined immunosuppressive effect of Foxp3 and IDO, we categorized each subject into one of four groups based on whether positive or negative for Foxp3 and IDO. The median proportion of Foxp3+ and IDO+ cells in the SLN in cancer patients were used the cut-off points. Subjects who were positive both for Foxp3 and IDO were almost exclusively those with nodal disease, (p = 0.007).
Similar articles
- Molecular analysis of melanoma-induced sentinel lymph node immune dysfunction.
Lee JH, Chen Y, Chan JL, Qian YW, Goydos JS. Lee JH, et al. Cancer Immunol Immunother. 2011 May;60(5):685-92. doi: 10.1007/s00262-011-0982-x. Epub 2011 Feb 16. Cancer Immunol Immunother. 2011. PMID: 21327637 Free PMC article. - Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients.
Speeckaert R, Vermaelen K, van Geel N, Autier P, Lambert J, Haspeslagh M, van Gele M, Thielemans K, Neyns B, Roche N, Verbeke N, Deron P, Speeckaert M, Brochez L. Speeckaert R, et al. Eur J Cancer. 2012 Sep;48(13):2004-11. doi: 10.1016/j.ejca.2011.09.007. Epub 2011 Oct 25. Eur J Cancer. 2012. PMID: 22033321 - Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis.
Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, Liu J, Ren X. Yu J, et al. Clin Dev Immunol. 2011;2011:469135. doi: 10.1155/2011/469135. Epub 2011 Oct 24. Clin Dev Immunol. 2011. PMID: 22110525 Free PMC article. - FDG-PET for axillary lymph node staging in primary breast cancer.
Crippa F, Gerali A, Alessi A, Agresti R, Bombardieri E. Crippa F, et al. Eur J Nucl Med Mol Imaging. 2004 Jun;31 Suppl 1:S97-102. doi: 10.1007/s00259-004-1531-z. Epub 2004 May 5. Eur J Nucl Med Mol Imaging. 2004. PMID: 15133635 Review. - Molecular analysis of sentinel lymph nodes and search for molecular signatures of the metastatic potential of breast cancer.
Hoon DS, Bernet L, Cano R, Viale G. Hoon DS, et al. Q J Nucl Med Mol Imaging. 2014 Jun;58(2):180-92. Q J Nucl Med Mol Imaging. 2014. PMID: 24835292 Review.
Cited by
- Synthesis and Biological Evaluation of Novel 2-Amino-1,4-Naphthoquinone Amide-Oxime Derivatives as Potent IDO1/STAT3 Dual Inhibitors with Prospective Antitumor Effects.
Huang RZ, Liang QL, Jing XT, Wang K, Zhang HY, Wang HS, Ma XL, Wei JH, Zhang Y. Huang RZ, et al. Molecules. 2023 Aug 19;28(16):6135. doi: 10.3390/molecules28166135. Molecules. 2023. PMID: 37630387 Free PMC article. - Involvement of the kynurenine pathway in breast cancer: updates on clinical research and trials.
Girithar HN, Staats Pires A, Ahn SB, Guillemin GJ, Gluch L, Heng B. Girithar HN, et al. Br J Cancer. 2023 Aug;129(2):185-203. doi: 10.1038/s41416-023-02245-7. Epub 2023 Apr 11. Br J Cancer. 2023. PMID: 37041200 Free PMC article. Review. - Reshaping the tumor microenvironment with oncolytic viruses, positive regulation of the immune synapse, and blockade of the immunosuppressive oncometabolic circuitry.
Nguyen TT, Shin DH, Sohoni S, Singh SK, Rivera-Molina Y, Jiang H, Fan X, Gumin J, Lang FF, Alvarez-Breckenridge C, Godoy-Vitorino F, Zhu L, Zheng WJ, Zhai L, Ladomersky E, Lauing KL, Alonso MM, Wainwright DA, Gomez-Manzano C, Fueyo J. Nguyen TT, et al. J Immunother Cancer. 2022 Jul;10(7):e004935. doi: 10.1136/jitc-2022-004935. J Immunother Cancer. 2022. PMID: 35902132 Free PMC article. - Nanomedicine as a Promising Tool to Overcome Immune Escape in Breast Cancer.
Navarro-Ocón A, Blaya-Cánovas JL, López-Tejada A, Blancas I, Sánchez-Martín RM, Garrido MJ, Griñán-Lisón C, Calahorra J, Cara FE, Ruiz-Cabello F, Marchal JA, Aptsiauri N, Granados-Principal S. Navarro-Ocón A, et al. Pharmaceutics. 2022 Feb 25;14(3):505. doi: 10.3390/pharmaceutics14030505. Pharmaceutics. 2022. PMID: 35335881 Free PMC article. Review. - Comprehensive Analysis to Identify the Epithelial-Mesenchymal Transition-Related Immune Signatures as a Prognostic and Therapeutic Biomarkers in Hepatocellular Carcinoma.
Wu G, Yang Y, Zhu Y, Li Y, Zhai Z, An L, Liu M, Zheng Y, Wang Y, Zhou Y, Guo Q. Wu G, et al. Front Surg. 2021 Oct 15;8:742443. doi: 10.3389/fsurg.2021.742443. eCollection 2021. Front Surg. 2021. PMID: 34722623 Free PMC article.
References
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2(6):389–400. - PubMed
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11(23):8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. - DOI - PubMed
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9(12):4404–4408. - PubMed
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials