Characterization of clinical Enterococcus faecalis small-colony variants - PubMed (original) (raw)
Case Reports
. 2009 Sep;47(9):2802-11.
doi: 10.1128/JCM.00485-09. Epub 2009 Jul 15.
Affiliations
- PMID: 19605585
- PMCID: PMC2738084
- DOI: 10.1128/JCM.00485-09
Case Reports
Characterization of clinical Enterococcus faecalis small-colony variants
Nele Wellinghausen et al. J Clin Microbiol. 2009 Sep.
Abstract
In this report, we present a clinical case of chronic aortic valve endocarditis caused by Enterococcus faecalis small-colony variants (SCVs), with ensuing characterization of the SCV phenotype in comparison to the clonally related normal phenotype with respect to alterations in microscopic and ultrastructural morphology, growth behavior, and metabolic pathways. In contrast to the normal phenotype, light and electron microscopy of the Enterococcus SCVs demonstrated the presence of heterogeneous cells of different sizes with aberrant shapes. Furthermore, SCVs showed excessive production of an intercellular substance and alterations in cell division displayed by a thick, coarse cell wall and incomplete, branched, and multiple cross walls without obvious cell separation. In addition, empty "ghost" cells were visible. In growth experiments, SCVs displayed an extended lag phase with delayed entrance into the stationary phase. Interestingly, SCV cells growing under aerobic conditions did not attain the growth and viability of the normal phenotype or those of SCVs growing under microaerobic conditions, suggesting impaired growth behavior and enhanced vulnerability in the presence of oxygen. By metabolite analysis, SCVs failed to produce significant amounts of acetate or lactate under aerobic growth conditions but were able to produce lactate under microaerobic growth conditions, implicating the induction of a fermentative metabolism. In conclusion, the observed structural alterations and changes in the cellular growth and metabolic pathways facilitated the survival of Enterococcus SCVs under microaerobic conditions in vitro and thus presumably in vivo during endocarditis.
Figures
FIG. 1.
Colony morphologies of E. faecalis wild-type and SCV phenotypes on CASO blood agar after 24 h of incubation.
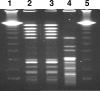
FIG. 2.
PFGE of E. faecalis wild-type and SCV clinical isolates after SmaI restriction. Lanes 1 and 5, 48.5- to 970-kb molecular weight marker (lambda λ_c_1857_S_am7; Bio-Rad, Germany); lane 2, wild-type phenotype; lane 3, SCV phenotype; lane 4, E. faecalis reference strain ATCC 29212.
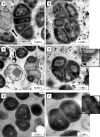
FIG. 3.
Gram staining of SCV (A) and wild-type (B) cells after 24 h of incubation on CASO blood agar.
FIG. 4.
SEM of SCV (A) and wild-type (B) cells. Magnification, ×20,000.
FIG. 5.
TEM of SCV (A to D) and wild-type (E and F) cells. White arrows point to coarse surfaces of SCV cells; black arrows indicate “ghost,” or empty, cells. Insets in panels D and F show details of cell wall structure at the same magnification (bars, 100 nm).
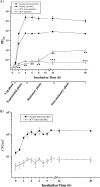
FIG. 6.
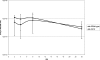
(A) Growth curves (OD600) of E. faecalis normal and SCV strains were determined in THY medium under aerobic and microaerobic conditions. Data are means ± standard errors of the means of values obtained in three independent experiments. **, P ≤ 0.005 for comparison to normal cells growing under microaerobic conditions; ***, P < 0.005 for comparison to SCV cells growing under microaerobic conditions (t test). (B) Viability assay for determination of the CFU counts (CFU ml−1) of E. faecalis normal and SCV strains. At different intervals, aliquots were removed, and CFU ml−1 was determined in duplicate. Data are means ± standard deviations of values obtained in two independent experiments.
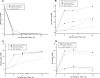
FIG. 7.
Determination of glucose concentrations and levels of metabolites (acetate, ammonia, and lactate) in culture supernatants under aerobic and microaerobic growth conditions. At different times, supernatants of E. faecalis normal and SCV strains, cultivated in THY medium, were analyzed for concentrations of glucose (A), acetate (B), ammonia (C), and lactate (D). Values are representative results of at least two independent experiments.
FIG. 8.
Intracellular replication of SCV and wild-type bacteria in MM6 cells. MM6 cells were infected at an MOI of 1:10, and cells were lysed 2, 4, 8, and 24 h after infection. The mean, minimal, and maximal values for four independent experiments are presented.
Similar articles
- Relapsing endocarditis caused by Enterococcus faecalis forming small colony variants.
Benes J, Dzupova O, Setina M, Feuereisl R, Svec P, Pantucek R. Benes J, et al. Scand J Infect Dis. 2013 Oct;45(10):800-3. doi: 10.3109/00365548.2013.800227. Epub 2013 Jul 1. Scand J Infect Dis. 2013. PMID: 23808721 - Characterization of an Enterococcus faecium small-colony variant isolated from blood culture.
Gröbner S, Beck J, Schaller M, Autenrieth IB, Schulte B. Gröbner S, et al. Int J Med Microbiol. 2012 Jan;302(1):40-4. doi: 10.1016/j.ijmm.2011.07.001. Epub 2011 Oct 2. Int J Med Microbiol. 2012. PMID: 21968291 - First Japanese case of infectious endocarditis due to Enterococcus faecalis small-colony variants.
Ogihara S, Saito R, Sawabe E, Hagihara M, Tohda S. Ogihara S, et al. J Infect Chemother. 2016 Oct;22(10):716-9. doi: 10.1016/j.jiac.2016.03.010. Epub 2016 Apr 16. J Infect Chemother. 2016. PMID: 27094238 - Pulmonic valve endocarditis.
Tariq M, Smego RA Jr, Soofi A, Islam N. Tariq M, et al. South Med J. 2003 Jun;96(6):621-3. doi: 10.1097/01.SMJ.0000047965.45842.A2. South Med J. 2003. PMID: 12938795 Review. - Prosthetic biologic valve endocarditis caused by a vancomycin-resistant (vanA) Enterococcus faecalis: case report.
Carfagna P, Tarasi A, Cassone M, Del Grosso MF, Bianco G, Venditti M. Carfagna P, et al. J Chemother. 2000 Oct;12(5):416-20. doi: 10.1179/joc.2000.12.5.416. J Chemother. 2000. PMID: 11128562 Review.
Cited by
- Antimicrobial Peptides can Enhance the Risk of Persistent Infections.
Berditsch M, Afonin S, Vladimirova T, Wadhwani P, Ulrich AS. Berditsch M, et al. Front Immunol. 2012 Aug 1;3:222. doi: 10.3389/fimmu.2012.00222. eCollection 2012. Front Immunol. 2012. PMID: 22870073 Free PMC article. No abstract available. - Tolerance and resistance of microbial biofilms.
Ciofu O, Moser C, Jensen PØ, Høiby N. Ciofu O, et al. Nat Rev Microbiol. 2022 Oct;20(10):621-635. doi: 10.1038/s41579-022-00682-4. Epub 2022 Feb 3. Nat Rev Microbiol. 2022. PMID: 35115704 Review. - Dwarfs in disguise: multiple spinal abscesses and spondylodiscitis caused by an Enterococcus faecium small-colony variant.
Höring S, Sobotta K, Schneider S, Löffler B, Rödel J. Höring S, et al. Access Microbiol. 2019 Mar 27;1(1):e000012. doi: 10.1099/acmi.0.000012. eCollection 2019. Access Microbiol. 2019. PMID: 32974494 Free PMC article. - Probiotic properties and adsorption of Enterococcus faecalis PSCT3-7 to vermiculite.
Kim JY, Awji EG, Park NH, Park JY, Kim JC, Lee SP, Suh JW, Park SC. Kim JY, et al. J Vet Sci. 2017 Mar 30;18(1):95-99. doi: 10.4142/jvs.2017.18.1.95. J Vet Sci. 2017. PMID: 27456777 Free PMC article. - Enterococcus faecalis infection activates phosphatidylinositol 3-kinase signaling to block apoptotic cell death in macrophages.
Zou J, Shankar N. Zou J, et al. Infect Immun. 2014 Dec;82(12):5132-42. doi: 10.1128/IAI.02426-14. Epub 2014 Sep 29. Infect Immun. 2014. PMID: 25267834 Free PMC article.
References
- Baddour, L. M., and G. D. Christensen. 1987. Prosthetic valve endocarditis due to small-colony staphylococcal variants. Rev. Infect. Dis. 91168-1174. - PubMed
- Chatterjee, I., A. Kriegeskorte, A. Fischer, S. Deiwick, N. Theimann, R. A. Proctor, G. Peters, M. Herrmann, and B. C. Kahl. 2008. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J. Bacteriol. 190834-842. - PMC - PubMed
- Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources