Antidepressants increase neural progenitor cells in the human hippocampus - PubMed (original) (raw)
Antidepressants increase neural progenitor cells in the human hippocampus
Maura Boldrini et al. Neuropsychopharmacology. 2009 Oct.
Abstract
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) increase neurogenesis in the dentate gyrus (DG) of rodents and nonhuman primates. We determined whether SSRIs or TCAs increase neural progenitor (NPCs) and dividing cells in the human DG in major depressive disorder (MDD). Whole frozen hippocampi from untreated subjects with MDD (N=5), antidepressant-treated MDD (MDDT, N=7), and controls (C, N=7) were fixed, sectioned, and immunostained for NPCs and dividing cell markers (nestin and Ki-67, respectively), NeuN and GFAP, in single and double labeling. NPC and dividing cell numbers in the DG were estimated by stereology. Clinical data were obtained by psychological autopsy, and by toxicological and neuropathological examination performed on all subjects. NPCs decreased with age (p = 0.034). Females had more NPCs than males (p = 0.023). Correcting for age and sex, MDDT receiving SSRIs had more NPCs than untreated MDD (p < or = 0.001) and controls (p < or = 0.001), NPCs were not different in SSRI- and TCA-treated MDDT (p = 0.169). Dividing cell number, unaffected by age or sex, was greater in MDDT receiving TCAs than in untreated MDD (p < or = 0.001), SSRI-treated MDD (p = 0.001), and controls (p < or = 0.001). The increase of NPCs and dividing cells in MDDT was localized to the rostral DG. MDDT had a larger DG volume compared with untreated MDD or controls (p = 0.009). Antidepressants increase NPC number in the anterior human DG. Whether this finding is critical or necessary for the antidepressants effect remains to be determined.
Conflict of interest statement
Disclosure/Conflict of Interest: The authors Maura Boldrini, Mark D. Underwood, Andrew J. Dwork, Gorazd B. Rosoklija and Victoria Arango declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Dr. Rene' Hen receives compensation as a consultant for BrainCells, Inc., PsychoGenics, Inc., and AstraZeneca in relation to the generation of novel antidepressants.
Dr. J. John Mann received grants from GlaxoSmithKline and Novartis.
Figures
Figure 1. Immunocytochemistry for NeuN, nestin, double labeling for NeuN and nestin and immunocytochemistry for Ki-67 in human dentate gyrus (DG) from a 57 years old female control (a-f) and a 75 years old male control (g-h) who were not on medication
(a) NeuN-immunoreactive (-IR) cells in the DG, hilus and CA regions of the hippocampus. (b) The subgranular zone (SGZ), granule cell layer (GCL) and Molecular layer (ML) are indicated; at higher magnification, the initial segments of the dendrites of the neurons of the hilus are clearly stained for NeuN (the same cells in the black box in a). (c) Nestin-IR cells (brown) along the SGZ of the DG exhibiting their characteristic multipolar appearance. Note one unipolar cell (arrow), which appears to have a higher level of differentiation, located within the GCL. Section is stained for Nissl with Cresyl Violet. (d) Differential interference contrast image of a nestin-IR cell, showing the immunostained perikarya and multiple processes, including one touching a blood vessel. (e) Double labeling of NeuN-IR granule cells (in black) and nestin-IR cells (in brown). Blood vessels are stained for nestin. (f) There is no co-labeling of Nestin and NeuN (the same cells in the black box in e). (g) Ki67-IR cells (nucleus in purple) in the GCL and SGZ of the DG. (k) Differential interference contrast image of the same cells in the black box in g. Four nuclei are labeled. After immunocytochemistry, sections were stained with eosin to label cytoplasm without interfering with the Ki-67 nuclear stain.
Figure 2. Nestin-immunoreactive (-IR) cells and vessels in the dentate gyrus (DG) from a 29 years old male with Major Depressive Disorder (MDD) who was not on medication (a) and a 31 years old male MDD who was treated with fluoxetine (b)
(a) The subgranular zone (SGZ) and granule cell layer (GCL) are indicated. Cells are stained for Nissl with Cresyl Violet. Nestin-IR cells and vessels appear in brown. (b) The fluoxetine-treated MDD shows more prominent nestin-IR cells processes and vessels compared to the untreated MDD (in a).
Figure 3. Human dentate gyrus (DG) treated with double-labeling immunocytochemistry for nestin and glial fibrillary acid protein (GFAP) and stained for Nissl substance. The subject was a 57-year-old female control with no medication
(a) The granular cell layer (GCL) appears in blue, GFAP-immunoreactive (-IR) cells stained with diaminobenzidine (DAB) appear brown. GFAP labels astrocytes in all subregions of the hippocampus (b) GFAP-IR cells are ubiquitously located throughout the hilus. (c) GFAP-IR cells located in the subgranular zone (SGZ) show processes that extend toward the molecular layer (ML), crossing the GCL. A GFAP-IR cell is seen in the SGZ near the lower edge of the picture (arrow). (d) GFAP-IR cells in the hilus at higher magnification. Astrocytes do not stain for nestin, but they do stain for GFAP (e, f) Nestin-IR cells (in black) appear along the SGZ (arrows).
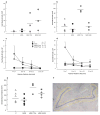
Figure 4. Progenitor cell (Nestin-IR) number decreased with increasing age
(a) Increasing age was associated with fewer progenitor cells (Nestin-IR) in subjects with Major Depressive Disorder (MDD) who received selective serotonin reuptake inhibitors (MDDT*SSRI) or tryciclics (MDDT*TCA). (b, c) In controls (C) and in untreated MDD subjects, the decrease in progenitor cell number with age did not reach statistical significance. (d) Age did not affect the number of dividing cells (Ki-67-IR) in any of the groups.
Figure 5. Neural progenitor and dividing cells are increased in the dentate gyrus (DG) of subjects with Major Depressive Disorder (MDD) who were treated with antidepressants (MDDT) compared to untreated MDDs and controls (C)
(a) Progenitor cells (Nestin-IR) were higher in MDDT treated with tryciclics (MDDT*TCA), as well as in MDDT treated with selective serotonin reuptake inhibitors (MDDT*SSRI) compared to untreated MDD and C (F = 984.105; df = 3,17; p ≤ 0.001). (b) Dividing cells (Ki-67-IR) were higher in MDD*TCA compared to the other groups (F = 16.243; df = 3,17; p ≤ 0.001). (c) Number of progenitor cells (Nestin-IR) in sections located in the anterior versus posterior hippocampal formation (error bars represent S.D.) (d) Number of dividing cells (Ki-67-IR) in sections located in the anterior versus posterior hippocampal formation (error bars represent S.D.) (e) DG volume in MDDT subjects treated with TCAs was larger compared to controls and MDD (F = 5.450; df = 3,17; p = 0.010) but not different from MDDT receiving SSRIs. (f) Image of one of the hippocampus sections showing the DG stained for Nissl (in blue). The outline defines the region of interest, within which cells were counted using stereology.
Similar articles
- Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression.
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Boldrini M, et al. Neuropsychopharmacology. 2013 May;38(6):1068-77. doi: 10.1038/npp.2013.5. Epub 2013 Jan 7. Neuropsychopharmacology. 2013. PMID: 23303074 Free PMC article. - Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression.
Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Boldrini M, et al. Biol Psychiatry. 2012 Oct 1;72(7):562-71. doi: 10.1016/j.biopsych.2012.04.024. Epub 2012 May 30. Biol Psychiatry. 2012. PMID: 22652019 Free PMC article. - Resilience Is Associated With Larger Dentate Gyrus, While Suicide Decedents With Major Depressive Disorder Have Fewer Granule Neurons.
Boldrini M, Galfalvy H, Dwork AJ, Rosoklija GB, Trencevska-Ivanovska I, Pavlovski G, Hen R, Arango V, Mann JJ. Boldrini M, et al. Biol Psychiatry. 2019 May 15;85(10):850-862. doi: 10.1016/j.biopsych.2018.12.022. Epub 2019 Jan 17. Biol Psychiatry. 2019. PMID: 30819514 Free PMC article. - Psychopharmacological Approaches for Neural Plasticity and Neurogenesis in Major Depressive Disorders.
Matar D, Serhan A, El Bilani S, Faraj RA, Hadi BA, Fakhoury M. Matar D, et al. Adv Exp Med Biol. 2024;1456:27-48. doi: 10.1007/978-981-97-4402-2_2. Adv Exp Med Biol. 2024. PMID: 39261422 Review. - Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise.
Micheli L, Ceccarelli M, D'Andrea G, Tirone F. Micheli L, et al. Brain Res Bull. 2018 Oct;143:181-193. doi: 10.1016/j.brainresbull.2018.09.002. Epub 2018 Sep 17. Brain Res Bull. 2018. PMID: 30236533 Review.
Cited by
- Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression.
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Boldrini M, et al. Neuropsychopharmacology. 2013 May;38(6):1068-77. doi: 10.1038/npp.2013.5. Epub 2013 Jan 7. Neuropsychopharmacology. 2013. PMID: 23303074 Free PMC article. - Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study.
Kurita M, Nishino S, Kato M, Numata Y, Sato T. Kurita M, et al. PLoS One. 2012;7(6):e39212. doi: 10.1371/journal.pone.0039212. Epub 2012 Jun 27. PLoS One. 2012. PMID: 22761741 Free PMC article. - Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood.
Ehlers CL, Liu W, Wills DN, Crews FT. Ehlers CL, et al. Neuroscience. 2013 Aug 6;244:1-15. doi: 10.1016/j.neuroscience.2013.03.058. Epub 2013 Apr 6. Neuroscience. 2013. PMID: 23567812 Free PMC article. - Adult Hippocampal Neurogenesis, Fear Generalization, and Stress.
Besnard A, Sahay A. Besnard A, et al. Neuropsychopharmacology. 2016 Jan;41(1):24-44. doi: 10.1038/npp.2015.167. Epub 2015 Jun 12. Neuropsychopharmacology. 2016. PMID: 26068726 Free PMC article. Review. - Aerobic Exercise as a Tool to Improve Hippocampal Plasticity and Function in Humans: Practical Implications for Mental Health Treatment.
Kandola A, Hendrikse J, Lucassen PJ, Yücel M. Kandola A, et al. Front Hum Neurosci. 2016 Jul 29;10:373. doi: 10.3389/fnhum.2016.00373. eCollection 2016. Front Hum Neurosci. 2016. PMID: 27524962 Free PMC article. Review.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1994.
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
- Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. - PubMed
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH064168/MH/NIMH NIH HHS/United States
- MH083862/MH/NIMH NIH HHS/United States
- R01 MH083862/MH/NIMH NIH HHS/United States
- R01 MH060877/MH/NIMH NIH HHS/United States
- MH40210/MH/NIMH NIH HHS/United States
- MH62185/MH/NIMH NIH HHS/United States
- P50 MH062185/MH/NIMH NIH HHS/United States
- MH64168/MH/NIMH NIH HHS/United States
- R01 MH083862-01A1/MH/NIMH NIH HHS/United States
- R01 MH064168-07/MH/NIMH NIH HHS/United States
- R01 MH040210/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous