The combined status of ATM and p53 link tumor development with therapeutic response - PubMed (original) (raw)
. 2009 Aug 15;23(16):1895-909.
doi: 10.1101/gad.1815309. Epub 2009 Jul 16.
Affiliations
- PMID: 19608766
- PMCID: PMC2725944
- DOI: 10.1101/gad.1815309
The combined status of ATM and p53 link tumor development with therapeutic response
Hai Jiang et al. Genes Dev. 2009.
Abstract
While the contribution of specific tumor suppressor networks to cancer development has been the subject of considerable recent study, it remains unclear how alterations in these networks are integrated to influence the response of tumors to anti-cancer treatments. Here, we show that mechanisms commonly used by tumors to bypass early neoplastic checkpoints ultimately determine chemotherapeutic response and generate tumor-specific vulnerabilities that can be exploited with targeted therapies. Specifically, evaluation of the combined status of ATM and p53, two commonly mutated tumor suppressor genes, can help to predict the clinical response to genotoxic chemotherapies. We show that in p53-deficient settings, suppression of ATM dramatically sensitizes tumors to DNA-damaging chemotherapy, whereas, conversely, in the presence of functional p53, suppression of ATM or its downstream target Chk2 actually protects tumors from being killed by genotoxic agents. Furthermore, ATM-deficient cancer cells display strong nononcogene addiction to DNA-PKcs for survival after DNA damage, such that suppression of DNA-PKcs in vivo resensitizes inherently chemoresistant ATM-deficient tumors to genotoxic chemotherapy. Thus, the specific set of alterations induced during tumor development plays a dominant role in determining both the tumor response to conventional chemotherapy and specific susceptibilities to targeted therapies in a given malignancy.
Figures
Figure 1.
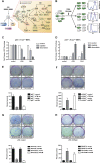
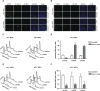
Functional integration of the ATM–Chk2 and p53 pathways determines the in vitro response to genotoxic chemotherapy. (A) Core components of the DNA damage response examined in this study. (B) Schematic representation of the GFP enrichment assay. Following drug treatments, the surviving population was assayed by flow cytometry to determine the percentage of GFP/shRNA-expressing cells. (C) Suppression of ATM and Chk2 in H-rasv12;p53−/− MEFs sensitizes cells to doxorubicin (1 μM) and cisplatin (1 μM). Bars indicate the mean of three experiments, with error bars indicating standard deviation. (D) Suppression of ATM and Chk2 in H-rasv12;Arf−/− MEFs confers resistance to doxorubicin (1 μM) and cisplatin (1 μM) in vitro (n = 3). Cells were treated as in C. (E–H) Clonogenic survival assays. (E) ATM inhibition (10 μM KU-55933 30 min prior to doxorubicin, top row) or shRNA-mediated ATM depletion (bottom row) results in reduced long-term survival in doxorubicin-treated (1 μM), p53-deficient MEFs. The bottom panel is a quantification of the results shown. (F) ATM inhibition or shRNA-mediated ATM depletion (as in E) confers a long-term survival benefit in doxorubicin-treated (1 μM), p53-proficient MEFs. (G) p53 mutant human cancer cells show increased doxorubicin sensitivity when ATM is inhibited. Cells were pretreated with KU-55933 (10 μM) for 30 min and colony survival assays were performed as in E. (H) p53-proficient human cancer cells show increased doxorubicin resistance when ATM is inhibited. Cells were treated as in G. Assays were performed in triplicate for each condition and representative images are shown.
Figure 2.
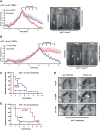
The in vivo effect of ATM and Chk2 pathway abrogation on tumor chemosensitivity is strictly dependent on p53 status. (A) ATM depletion sensitizes p53-deficient tumors to the cytotoxic effects of doxorubicin in vivo. H-RasV12_-transformed p53−/− MEFs expressing either a control shRNA (blue) or ATM-specific shRNA (red) were subcutaneously injected into the flanks of NCR_nu/nu mice (n = 4 for each experimental group). Arrows indicate the timing of intraperitoneal (i.p.) doxorubicin administrations. Asterisks indicate significant size difference (Student's _t_-test, two-tailed, P < 0.05). (Right panels) Maintenance of GFP expression in tumors was verified at the termination point. (B) Depletion of ATM in tumors arising from Arf−/−;H-RasV12 MEFs confers resistance to doxorubicin in vivo (n = 4 for each experimental group). Animals were subcutaneously injected with Arf−/−;H-RasV12 MEFs expressing either a control shRNA (blue) or an ATM-specific hairpin (red). Doxorubicin was administered i.p. at the indicated times and tumor growth was monitored as in A. (Right panels) Maintenance of GFP expression in tumors was verified at the termination point. (C) Suppression of ATM or Chk2 in Eμ-Myc;Arf−/− mouse lymphoma confers resistance to doxorubicin in vivo. Lymphoma cells were transduced with vector control, shATM, or shChk2; sorted for GFP; and injected into recipient mice. Resulting lymphomas were treated with 10 mg/kg doxorubicin. Tumor-free survival displayed in Kaplan-Meier format. n = 10 for shATM and shChk2; n = 11 for vector control. (D) Representative images of lymphoma burden before treatment and 5 d post-therapy. (E) ATM depletion sensitizes p53-deficient lymphomas to the cytotoxic effects of cyclophosphamide in vivo. p53-null lymphomas were transduced and injected as in C. n = 8 for both vector and shATM.
Figure 3.
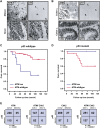
Combined ATM and p53 status is a key determinant for survival of breast cancer patients treated with DNA-damaging chemotherapy. (A,B) Examples of ATM, p53, and Chk2 aberrations in human tumors. Immunohistochemical staining with antibodies against the indicated proteins, showing parallel sections from a large-cell lung carcinoma (NSCLC, with loss of ATM but normal p53), small-cell lung carcinoma (SCLC, with normal ATM pattern but overabundant, mutant p53), and two colon carcinomas—one with loss of ATM and normal p53, the other with normal Chk2 but aberrant p53. The letters “t” and “s” on the images indicate the positions of tumor nests and stromal cells, respectively, to highlight the selective absence of ATM in the carcinoma cells. (C,D) Kaplan-Meier curves showing overall survival in 93 breast cancer patients (omitting those treated with IR). (C) ATM deficiency in a p53 wild-type background correlates with poor patient survival relative to tumors with wild-type ATM and p53 (P = 0.0059). (D) Relative survival of patients with tumors showing ATM deficiency on a p53 mutant background versus mutant p53 alone (P = 0.32). (E) The combined inactivation of ATM/Chk2 and p53 is underrepresented in human cancers. (Far left) ATM versus p53 status in 400 tumors examined for both proteins. (Middle left) ATM/Chk2 versus p53 status in 279 tumors examined for all three proteins. (Middle right) Chk2 versus p53 status in 335 tumors examined for both proteins. (Far right) ATM/Chk2 versus p53 status in 456 tumors examined for p53 and either ATM or Chk2 status.
Figure 4.
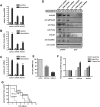
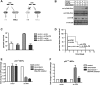
Depletion of ATM or Chk2 strongly reduces p53-mediated induction of proapoptotic genes but preserves the induction of p53-dependent cell cycle-regulating genes. (A–D) RNAi-mediated knockdown of ATM or Chk2 strongly reduces doxorubicin-induced Puma and Noxa expression, but only mildly impairs p21 mRNA levels in p53-proficient MEFs. Cells expressing ATM or Chk2-specific shRNAs were treated with 1 μM doxorubicin and mRNA levels of Noxa (A), Puma (B), and p21 (C) were analyzed (n = 3). (D) Depletion of ATM or Chk2 in p53-proficient MEFs abolishes doxorubicin-induced Puma and Noxa protein levels. Protein levels of the p53 target genes p21 and Gadd45α remains largely unchanged in ATM- or Chk2-depleted p53-proficient cells treated with doxorubicin (1 μM). Noxa, Puma, and p21 were not detectable in p53-deficient MEFs, while Gadd45α was present, but at reduced levels. (E) shRNA-mediated suppression of Puma and Noxa. Eμ-Myc cells were transduced with control, shPuma, or shNoxa shRNAs; sorted with for GFP expression; and treated with 15 ng/mL doxorubicin for 12 h. mRNA levels of Puma and Noxa were analyzed by RT–PCR (n = 3). (F) Suppression of Puma and Noxa in Eμ-Myc cells confers resistance to doxorubicin in vitro (n = 3). Cells were analyzed for GFP percentage after doxorubicin treatment. Bars indicate the mean of three experiments ±SEM. (G) Suppression of Puma, but not Noxa, in lymphomas arising from Eμ-Myc cells confers resistance to doxorubicin in vivo. Experiments were carried out as described in Figure 2C, with n = 10 for shPuma, n = 11 for vector control, and n = 5 for shNoxa.
Figure 5.
Depletion of ATM specifically sensitizes p53-deficient MEFs to doxorubicin-induced mitotic catastrophe. p53-deficient (A) and p53-proficient (B) MEFs stably expressing ATM-specific shRNA were either mock-treated or exposed to 1 μM doxorubicin and stained with antibodies detecting γ-H2AX, cleaved caspase-3, pHH3, and Hoechst DNA dye. Costaining with γ-H2AX, cleaved caspase-3, and pHH3 was interpreted as mitotic catastrophe (indicated by arrowheads). (C,D) ATM depletion in p53-deficient MEFs prevents the engagement of a functional G2/M checkpoint following doxorubicin. p53−/− cells expressing control shRNA mounted a robust G2/M arrest in response to nocodazole, as evidenced by an accumulation of 4N cells (monitored by PI staining) and a lack of pHH3 staining. In contrast, ∼30% of ATM-depleted p53-null cells entered mitosis, indicating a bypass of the doxorubicin-induced G2/M arrest in these cells. (E,F) ATM depletion in p53-proficient MEFs does not abrogate the G2/M checkpoint following doxorubicin. Asynchronously growing ATM or control shRNA-expressing p53+/+ MEFs were treated as in C and examined for DNA content by flow cytmoetry. These cells retained a doxorubicin-induced G2/M checkpoint, as evidenced by the accumulation of a largely pHH3-negative 4N population in both the control and ATM shRNA-expressing cells.
Figure 6.
A synthetic lethal interaction between ATM and DNA-PKcs in cancer cells. (A) A diagram depicting the DNA repair pathways used for DSB repair. ATM-mediated HR represents a high-fidelity repair mechanism for DSB repair. In the absence of a functional HR pathway, cells rely on the error-prone NHEJ pathway, which requires DNA-PKcs activity. (B) Immunoblot analysis of DNA-PKcs activation in MEFs transduced with an empty vector or ATM-specific shRNA. RNAi-mediated ATM depletion results in increased DNA-PKcs phosphorylation after doxorubicin exposure (1 μM). (C,D) DNA-PKcs suppression resensitizes p53-proficient ATM-depleted Eμ-Myc cells to doxorubicin in vitro (C) and in vivo (D). In C, cells of all four indicated treatments were incubated with 10 ng/mL doxorubicin. The percent cell survival following treatment is shown as mean ± SEM (n = 3). In D, experiments were carried out as described in Figure 2C. n = 8 for both experimental groups. (E,F) Pharmacological inhibition of DNA-PKcs selectively sensitizes p53-proficient ATM shRNA-expressing MEFs to the cytotoxic effects of doxorubicin. MEFs were transduced and treated as indicated and cellular survival was monitored using the flow cytometry-based GFP competition assay. (E) DNA-PKcs inhibition had no significant effect on the survival of p53−/− MEFs or ATM-depleted p53−/− MEFs. (F) DNA-PKcs inhibition selectively sensitized p53+/+ MEFs expressing an ATM shRNA to doxorubicin.
Figure 7.
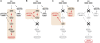
ATM acts as a binary switch to control the contribution of p53 signaling to the DNA damage response. The DNA damage response can be subdivided into three major functional components—cell cycle arrest, DNA repair, and apoptosis. (A) In p53-proficient cancer cells, ATM signaling contributes largely to apoptosis (highlighted in orange). (B) Loss of p53 in tumor cells dramatically reduces apoptosis and redirects ATM signaling to mediate a robust cell cycle (highlighted in orange). In addition, ATM contributes to HR-mediated DSB repair. The net result of this rewired ATM signaling is increased cellular survival in response to DNA damage. (C) Loss of ATM dramatically attenuates apoptotic signaling through p53 and instead promotes a p53-mediated cell cycle arrest (highlighted in orange). ATM-deficient cancer cells expressing functional p53 rely on the DNA-PKcs-mediated NHEJ pathway to repair chemotherapy-induced DSBs (highlighted in orange). Pharmacological abrogation of DNA-PKcs signaling selectively sensitizes ATM-deficient p53-expressing cancer cells to the cytotoxic effects of DNA-damaging chemotherapy. (D) The combined loss of ATM and p53, albeit rare in human tumors, prevents the execution of functional cell cycle checkpoints and promotes death of chemotherapy-treated cancer cells due to mitotic catastrophe. In addition, therapeutic targeting of ATM in p53-deficient cancer cells results in a dramatically increased chemosensitivity of p53-deficient cancer cells.
Similar articles
- ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis.
Pabla N, Huang S, Mi QS, Daniel R, Dong Z. Pabla N, et al. J Biol Chem. 2008 Mar 7;283(10):6572-83. doi: 10.1074/jbc.M707568200. Epub 2007 Dec 27. J Biol Chem. 2008. PMID: 18162465 - Role of autophagy in chemoresistance: regulation of the ATM-mediated DNA-damage signaling pathway through activation of DNA-PKcs and PARP-1.
Yoon JH, Ahn SG, Lee BH, Jung SH, Oh SH. Yoon JH, et al. Biochem Pharmacol. 2012 Mar 15;83(6):747-57. doi: 10.1016/j.bcp.2011.12.029. Epub 2011 Dec 29. Biochem Pharmacol. 2012. PMID: 22226932 - Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin.
Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Shi Y, et al. BMC Cancer. 2012 Dec 4;12:571. doi: 10.1186/1471-2407-12-571. BMC Cancer. 2012. PMID: 23211021 Free PMC article. - The ATM protein kinase and cellular redox signaling: beyond the DNA damage response.
Ditch S, Paull TT. Ditch S, et al. Trends Biochem Sci. 2012 Jan;37(1):15-22. doi: 10.1016/j.tibs.2011.10.002. Epub 2011 Nov 11. Trends Biochem Sci. 2012. PMID: 22079189 Free PMC article. Review. - Targeting DNA checkpoint kinases in cancer therapy.
Zhou BB, Anderson HJ, Roberge M. Zhou BB, et al. Cancer Biol Ther. 2003 Jul-Aug;2(4 Suppl 1):S16-22. Cancer Biol Ther. 2003. PMID: 14508077 Review.
Cited by
- Combined Inhibition of MEK and Plk1 Has Synergistic Antitumor Activity in NRAS Mutant Melanoma.
Posch C, Cholewa BD, Vujic I, Sanlorenzo M, Ma J, Kim ST, Kleffel S, Schatton T, Rappersberger K, Gutteridge R, Ahmad N, Ortiz/Urda S. Posch C, et al. J Invest Dermatol. 2015 Oct;135(10):2475-2483. doi: 10.1038/jid.2015.198. Epub 2015 May 27. J Invest Dermatol. 2015. PMID: 26016894 Free PMC article. - Targeting radiation-resistant hypoxic tumour cells through ATR inhibition.
Pires IM, Olcina MM, Anbalagan S, Pollard JR, Reaper PM, Charlton PA, McKenna WG, Hammond EM. Pires IM, et al. Br J Cancer. 2012 Jul 10;107(2):291-9. doi: 10.1038/bjc.2012.265. Epub 2012 Jun 19. Br J Cancer. 2012. PMID: 22713662 Free PMC article. - Nuclear Proteomics of Induced Leukemia Cell Differentiation.
Novikova S, Tolstova T, Kurbatov L, Farafonova T, Tikhonova O, Soloveva N, Rusanov A, Archakov A, Zgoda V. Novikova S, et al. Cells. 2022 Oct 14;11(20):3221. doi: 10.3390/cells11203221. Cells. 2022. PMID: 36291090 Free PMC article. - The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance.
Bouwman P, Jonkers J. Bouwman P, et al. Nat Rev Cancer. 2012 Sep;12(9):587-98. doi: 10.1038/nrc3342. Nat Rev Cancer. 2012. PMID: 22918414 Review. - Tyrosine 370 phosphorylation of ATM positively regulates DNA damage response.
Lee HJ, Lan L, Peng G, Chang WC, Hsu MC, Wang YN, Cheng CC, Wei L, Nakajima S, Chang SS, Liao HW, Chen CH, Lavin M, Ang KK, Lin SY, Hung MC. Lee HJ, et al. Cell Res. 2015 Feb;25(2):225-36. doi: 10.1038/cr.2015.8. Epub 2015 Jan 20. Cell Res. 2015. PMID: 25601159 Free PMC article.
References
- Austen B, Skowronska A, Baker C, Powell JE, Gardiner A, Oscier D, Majid A, Dyer M, Siebert R, Taylor AM, et al. Mutation status of the residual ATM allele is an important determinant of the cellular response to chemotherapy and survival in patients with chronic lymphocytic leukemia containing an 11q deletion. J Clin Oncol. 2007;25:5448–5457. - PubMed
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. - PubMed
- Bartek J, Iggo R, Gannon J, Lane DP. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990;5:893–899. - PubMed
- Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5:792–804. - PubMed
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES015339/ES/NIEHS NIH HHS/United States
- R01 ES15339/ES/NIEHS NIH HHS/United States
- 1 R01 CA128803-01/CA/NCI NIH HHS/United States
- R01 CA128803/CA/NCI NIH HHS/United States
- U54 CA112967/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous