MYCN promotes the expansion of Phox2B-positive neuronal progenitors to drive neuroblastoma development - PubMed (original) (raw)
MYCN promotes the expansion of Phox2B-positive neuronal progenitors to drive neuroblastoma development
Goleeta Alam et al. Am J Pathol. 2009 Aug.
Abstract
Amplification of the oncogene MYCN is a tumorigenic event in the development of a subset of neuroblastomas that commonly consist of undifferentiated or poorly differentiated neuroblasts with unfavorable clinical outcome. The cellular origin of these neuroblasts is unknown. Additionally, the cellular functions and target cells of MYCN in neuroblastoma development remain undefined. Here we examine the cell types that drive neuroblastoma development in TH-MYCN transgenic mice, an animal model of the human disease. Neuroblastoma development in these mice begins with hyperplastic lesions in early postnatal sympathetic ganglia. We show that both hyperplasia and primary tumors are composed predominantly of highly proliferative Phox2B(+) neuronal progenitors. MYCN induces the expansion of these progenitors by both promoting their proliferation and preventing their differentiation. We further identify a minor population of undifferentiated nestin(+) cells in both hyperplastic lesions and primary tumors that may serve as precursors of Phox2B(+) neuronal progenitors. These findings establish the identity of neuroblasts that characterize the tumor phenotype and suggest a cellular pathway by which MYCN can promote neuroblastoma development.
Figures
Figure 1
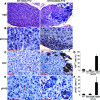
Early postnatal sympathetic ganglia of TH-MYCN mice contain multiple clusters of proliferating cells. Sections of SCG from postnatal day 12 (P12) wild-type and TH-MYCN mice were stained with H&E (A), anti-MYCN (B), anti-Ki-67 (C), or anti-pHH3 (E). Wild-type SCG consist of mainly large neurons and small glial satellite cells (A) that express no detectable levels of MYCN (B), and the proliferating cells marked by Ki-67 (C) or pHH3 (E, arrowhead) were evenly distributed. By contrast, TH-MYCN SCG contain multiple clusters of small round blue cells (A) that express MYCN (B) and are in a state of active proliferation (C, E). Scale bars = 100 μm. D, F: Quantitative analysis of ganglionic cells expressing either Ki-67 (D) or pHH3 (F) in wild-type SCG (n = 4) and in non-hyperplastic (Non-Hyper) and hyperplastic (Hyper) regions of TH-MYCN SCG (n = 4). Data are presented as means ± SD.
Figure 2
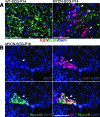
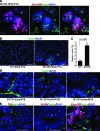
Hyperplastic lesions are composed predominantly of proliferating Phox2B+ cells. A: Sections of SCG from P14 wild-type and TH-MYCN mice were stained with anti-Ki-67 (red) and anti-BLBP (green), an early marker for glial cells. Most of the Ki-67+ cells in wild-type SCG also express BLBP, whereas Ki-67+ cells in hyperplastic lesions (arrows) are negative for BLBP. B: Immunofluorescent staining of a representative SCG section from a P16 TH-MYCN mouse shows a hyperplastic lesion (arrow) consisting predominantly of cells expressing both Ki-67 (red) and Phox2B (green). Nuclei were stained with DAPI (blue). Scale bars = 100 μm.
Figure 3
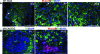
Phox2B+ hyperplastic cells are arrested in neuronal differentiation. A: Sections of SCG from P4, P7, and P14 wild-type mice were stained with anti-Phox2B (red) and anti-TH (green), a late marker for sympathetic neurons. Most of the Phox2B+ cells display the morphology of mature neurons and express TH. Only a few Phox2B+ cells in P4 and P7 SCG stained negatively for TH (arrows). B: Sections of SCG from P12 and P16 TH-MYCN mice were stained with anti-Phox2B (red) and/or anti-TH (green). Most of the hyperplastic cells are Phox2B+TH− neuronal progenitors. Nuclei were stained with DAPI (blue). Scale bars = 100 μm.
Figure 4
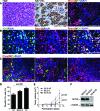
Phox2B+ progenitor cells are the major cellular component of mouse primary neuroblastoma tumors and xenograft tumors derived from human neuroblastoma cell lines. A: Sections of neuroblastoma tumors from TH-MYCN mice were stained with H&E, anti-Ki-67, or anti-Phox2B (red) and anti-TH (green). Nuclei were stained with DAPI (blue). The small round blue tumor cells are organized in nests surrounded by thin fibrovascular septa (H&E), and most of them express Ki-67 and Phox2B, but are negative for TH. B: Immunofluorescent staining of a representative mouse neuroblastoma section for Ki-67 (green) and Phox2B (red). Nuclei were stained with DAPI (blue). C: Sections of xenograft tumors derived from human neuroblastoma cell lines were stained with anti-Phox2B (red) and anti-TH (green). Nuclei were stained with DAPI (blue). Most of the tumor cells express Phox2B. SK-N-DZ and BE(2)-C xenografts also contain significant numbers of TH+ cells. The cells stained yellow in SK-N-DZ xenograft section are probably necrotic cells. Scale bars = 100 μm (A-C). D, E: Quantification of Phox2B+TH− progenitor cells (D) in xenograft tumors of different human neuroblastoma cell lines reveals a positive correlation with the tumor growth rates (E). Data are presented as means ± SD and analyzed with two-tailed Student’s _t_-test with P values indicated. F: Immunoblot analysis of MYCN protein levels in human neuroblastoma cell lines. α-tubulin levels are shown as loading control.
Figure 5
Hyperplasia and primary neuroblastoma tumors contain a minor population of nestin+ progenitor cells. A: Sections of hyperplastic lesions were stained with anti-Phox2B (red), anti-nestin (green), and DAPI (blue), and examined with a confocal microscope. Arrows and asterisks indicate nestin+ and nestin+Phox2B+ cells, respectively. B: Sections of wild-type and MYCN sympathetic ganglia at P14 were stained with anti-nestin (green) and DAPI (blue). The arrow indicates a hyperplastic lesion in the MYCN SCG. C: Quantitative analysis showing a fourfold increase in the number of nestin+ cells in TH-MYCN SCG with hyperplastic lesions compared with age-matched wild-type SCG (P14 to P16). Data are presented as means ± SD and analyzed by two-tailed Student’s _t_-test with the P value indicated. D, E: Sections of representative primary neuroblastoma tumors from TH-MYCN mice were stained with anti-nestin (green) alone (D) or with anti-TH (red), anti-BLBP (red) or anti-Phox2B (red) (E). Nuclei were stained with DAPI (blue). Nestin+ tumor cells are negative for TH and BLBP, and some of them express Phox2B (arrowheads). Scale bars: 10 μm (A); 50 μm (B, D, and E).
Figure 6
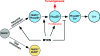
A simplified schematic diagram for the cellular pathway in MYCN-induced neuroblastoma development. See text for discussion.
Similar articles
- MYCN and ALKF1174L are sufficient to drive neuroblastoma development from neural crest progenitor cells.
Schulte JH, Lindner S, Bohrer A, Maurer J, De Preter K, Lefever S, Heukamp L, Schulte S, Molenaar J, Versteeg R, Thor T, Künkele A, Vandesompele J, Speleman F, Schorle H, Eggert A, Schramm A. Schulte JH, et al. Oncogene. 2013 Feb 21;32(8):1059-65. doi: 10.1038/onc.2012.106. Epub 2012 Apr 9. Oncogene. 2013. PMID: 22484425 - Activated ALK collaborates with MYCN in neuroblastoma pathogenesis.
Zhu S, Lee JS, Guo F, Shin J, Perez-Atayde AR, Kutok JL, Rodig SJ, Neuberg DS, Helman D, Feng H, Stewart RA, Wang W, George RE, Kanki JP, Look AT. Zhu S, et al. Cancer Cell. 2012 Mar 20;21(3):362-73. doi: 10.1016/j.ccr.2012.02.010. Cancer Cell. 2012. PMID: 22439933 Free PMC article. - Proliferation and Survival of Embryonic Sympathetic Neuroblasts by MYCN and Activated ALK Signaling.
Kramer M, Ribeiro D, Arsenian-Henriksson M, Deller T, Rohrer H. Kramer M, et al. J Neurosci. 2016 Oct 5;36(40):10425-10439. doi: 10.1523/JNEUROSCI.0183-16.2016. J Neurosci. 2016. PMID: 27707976 Free PMC article. - The MYCN oncogene and differentiation in neuroblastoma.
Westermark UK, Wilhelm M, Frenzel A, Henriksson MA. Westermark UK, et al. Semin Cancer Biol. 2011 Oct;21(4):256-66. doi: 10.1016/j.semcancer.2011.08.001. Epub 2011 Aug 9. Semin Cancer Biol. 2011. PMID: 21849159 Review. - The MYCN oncoprotein as a drug development target.
Lu X, Pearson A, Lunec J. Lu X, et al. Cancer Lett. 2003 Jul 18;197(1-2):125-30. doi: 10.1016/s0304-3835(03)00096-x. Cancer Lett. 2003. PMID: 12880971 Review.
Cited by
- Expansion of a neural crest gene signature following ectopic MYCN expression in sympathoadrenal lineage cells in vivo.
Ibarra-García-Padilla R, Nambiar A, Hamre TA, Singleton EW, Uribe RA. Ibarra-García-Padilla R, et al. PLoS One. 2024 Sep 18;19(9):e0310727. doi: 10.1371/journal.pone.0310727. eCollection 2024. PLoS One. 2024. PMID: 39292691 Free PMC article. - Caffeine Supplementation and FOXM1 Inhibition Enhance the Antitumor Effect of Statins in Neuroblastoma.
Tran GB, Ding J, Ye B, Liu M, Yu Y, Zha Y, Dong Z, Liu K, Sudarshan S, Ding HF. Tran GB, et al. Cancer Res. 2023 Jul 5;83(13):2248-2261. doi: 10.1158/0008-5472.CAN-22-3450. Cancer Res. 2023. PMID: 37057874 Free PMC article. - Deciphering the Role of p53 and TAp73 in Neuroblastoma: From Pathogenesis to Treatment.
Almeida J, Mota I, Skoda J, Sousa E, Cidade H, Saraiva L. Almeida J, et al. Cancers (Basel). 2022 Dec 16;14(24):6212. doi: 10.3390/cancers14246212. Cancers (Basel). 2022. PMID: 36551697 Free PMC article. Review. - MYCN and Metabolic Reprogramming in Neuroblastoma.
Bansal M, Gupta A, Ding HF. Bansal M, et al. Cancers (Basel). 2022 Aug 25;14(17):4113. doi: 10.3390/cancers14174113. Cancers (Basel). 2022. PMID: 36077650 Free PMC article. Review. - Recent advances in the developmental origin of neuroblastoma: an overview.
Ponzoni M, Bachetti T, Corrias MV, Brignole C, Pastorino F, Calarco E, Bensa V, Giusto E, Ceccherini I, Perri P. Ponzoni M, et al. J Exp Clin Cancer Res. 2022 Mar 11;41(1):92. doi: 10.1186/s13046-022-02281-w. J Exp Clin Cancer Res. 2022. PMID: 35277192 Free PMC article. Review.
References
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
- LaBonne C, Bronner-Fraser M. Induction and patterning of the neural crest, a stem cell-like precursor population. J Neurobiol. 1998;36:175–189. - PubMed
- Le Douarin NM, Dupin E. Cell lineage analysis in neural crest ontogeny. J Neurobiol. 1993;24:146–161. - PubMed
- Kirby ML, Gilmore SA. A correlative histofluorescence and light microscopic study of the formation of the sympathetic trunks in chick embryos. Anat Rec. 1976;186:437–449. - PubMed
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases