Akt phosphorylates both Tsc1 and Tsc2 in Drosophila, but neither phosphorylation is required for normal animal growth - PubMed (original) (raw)
Akt phosphorylates both Tsc1 and Tsc2 in Drosophila, but neither phosphorylation is required for normal animal growth
Sibylle Schleich et al. PLoS One. 2009.
Abstract
Akt, an essential component of the insulin pathway, is a potent inducer of tissue growth. One of Akt's phosphorylation targets is Tsc2, an inhibitor of the anabolic kinase TOR. This could account for part of Akt's growth promoting activity. Although phosphorylation of Tsc2 by Akt does occur in vivo, and under certain circumstances can lead to reduced Tsc2 activity, the functional significance of this event is unclear since flies lacking Akt phosphorylation sites on Tsc2 are viable and normal in size and growth rate. Since Drosophila Tsc1, the obligate partner of Tsc2, has an Akt phosphorylation motif that is not conserved in mammals, we investigate here whether Akt redundantly phosphorylates the Tsc complex on Tsc1 and Tsc2. We provide evidence that Akt phosphorylates Tsc1 at Ser533. We show that flies lacking Akt phosphorylation sites on Tsc1 alone, or on both Tsc1 and Tsc2 concurrently, are viable and normal in size. This shows that phosphorylation of the Tsc1/2 complex by Akt is not required for Akt to activate TORC1 and to promote tissue growth in Drosophila.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
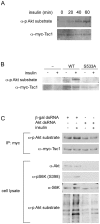
Figure 1. Drosophila Tsc1 is phosphorylated by Akt on Ser533.
(A) Phosphorylation of Tsc1 increases with insulin treatment. S2 cells transfected with constructs to express myc-Tsc1 and His-Tsc2 were treated without insulin (0 min) or with insulin (10 µg/mL) for indicated times (20, 40 or 60 min). Cells were then lysed and myc-Tsc1 immunoprecipitated using anti-myc antibody. Immunoprecipitates were probed with anti-myc antibody as a loading control, and anti-Phospho-(Ser/Thr) Akt Substrate antibody to detect phosphorylation of Tsc1. (Ser533 is part of an Akt phosphorylation consensus motif). (B) Tsc1 is phosphorylated on Ser533 in response to insulin treatment. Untransfected S2 cells (-) or S2 cells transfected with constructs to express either myc-Tsc1WT (“WT”) or myc-Tsc1S533A (“S533A”) together with His-Tsc2 were treated with or without insulin (10 µg/mL) for 1 hour prior to lysis and immunoprecipitation with anti-myc antibody. Immunoprecipitates were probed with anti-myc antibody as a loading control, and anti-Phospho-(Ser/Thr) Akt Substrate antibody to detect phosphorylation of Tsc1. (C) Knockdown of Akt abrogates the increase in phosphorylation of Tsc1 on Ser533 induced by insulin treatment. S2 cells transfected with expression constructs for myc-Tsc1WT and His-Tsc2 were treated with control dsRNA or Akt dsRNA for 4 days prior to insulin treatment (10 µg/mL for 1 hour), lysis and anti-myc immunoprecipitation. Immunoprecipitates were probed with anti-myc as a control and anti Phospho-(Ser/Thr) Akt Substrate antibody to detect phosphorylation of Tsc1 Ser533. Despite efficient knockdown of Akt (seen by lack of Akt protein and S6K phosphorylation in lanes 3 and 4), anti Phospho-(Ser/Thr) Akt Substrate antibody displays background binding in total cell lysates, as previously reported also by others.
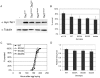
Figure 2. Flies lacking Akt phosphorylation sites on Tsc1 and Tsc2 are viable and normal in size.
(A) Expression levels of myc-Tsc1 in fly lines homozygous for the Tsc129 mutation, rescued by ubiquitous expression of Tsc1WT, Tsc1S533A or Tsc1S533D, or flies homozygous for both the Tsc129 and Tsc2192 mutations rescued to viability by ubiquitous expression of both Tsc1S533A and Tsc2T437A/S924A/T1054A/T1518A (“Tsc1S533A,Tsc24A”). (B,C,D) Survival rates (B), pupation curves (C) and relative adult wing sizes (D) of animals seeded as L1 larvae under controlled growth conditions for genotypes w1118 (“w1118”), Tsc129 homozygotes rescued by ubiquitous expression of Tsc1WT (“WT”), Tsc1S533A (“S533A”) or Tsc1S533D(“S533D”), or flies homozygous for both the Tsc129 and Tsc2192 mutations rescued to viability by ubiquitous expression of both Tsc1S533A and Tsc2T437A/S924A/T1054A/T1518A (“double”).
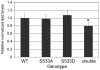
Figure 3. Flies lacking Akt phosphorylation sites on both Tsc1 and Tsc2 are slightly lean.
Triglyceride levels normalized to total body protein for Tsc129 homozygotes rescued by ubiquitous expression of Tsc1WT (“WT”), Tsc1S533A (“S533A”) or Tsc1S533D(“S533D”), or flies homozygous for both the Tsc129 and Tsc2192 mutations rescued to viability by expression of both Tsc1S533A and Tsc2T437A/S924A/T1054A/T1518A (“double”). * indicates statistical significance (ttest = 0.01).
Similar articles
- Akt regulates growth by directly phosphorylating Tsc2.
Potter CJ, Pedraza LG, Xu T. Potter CJ, et al. Nat Cell Biol. 2002 Sep;4(9):658-65. doi: 10.1038/ncb840. Nat Cell Biol. 2002. PMID: 12172554 - Tuberous sclerosis complex regulates Drosophila neuromuscular junction growth via the TORC2/Akt pathway.
Natarajan R, Trivedi-Vyas D, Wairkar YP. Natarajan R, et al. Hum Mol Genet. 2013 May 15;22(10):2010-23. doi: 10.1093/hmg/ddt053. Epub 2013 Feb 7. Hum Mol Genet. 2013. PMID: 23393158 - A complex interplay between Akt, TSC2 and the two mTOR complexes.
Huang J, Manning BD. Huang J, et al. Biochem Soc Trans. 2009 Feb;37(Pt 1):217-22. doi: 10.1042/BST0370217. Biochem Soc Trans. 2009. PMID: 19143635 Free PMC article. Review. - Insulin like growth factor-1-induced phosphorylation and altered distribution of tuberous sclerosis complex (TSC)1/TSC2 in C2C12 myotubes.
Miyazaki M, McCarthy JJ, Esser KA. Miyazaki M, et al. FEBS J. 2010 May;277(9):2180-91. doi: 10.1111/j.1742-4658.2010.07635.x. FEBS J. 2010. PMID: 20412061 Free PMC article. - Rhebbing up mTOR: new insights on TSC1 and TSC2, and the pathogenesis of tuberous sclerosis.
Kwiatkowski DJ. Kwiatkowski DJ. Cancer Biol Ther. 2003 Sep-Oct;2(5):471-6. doi: 10.4161/cbt.2.5.446. Cancer Biol Ther. 2003. PMID: 14614311 Review.
Cited by
- Regulation of Body Size and Growth Control.
Texada MJ, Koyama T, Rewitz K. Texada MJ, et al. Genetics. 2020 Oct;216(2):269-313. doi: 10.1534/genetics.120.303095. Genetics. 2020. PMID: 33023929 Free PMC article. Review. - Drosophila melanogaster as a Model for Diabetes Type 2 Progression.
Álvarez-Rendón JP, Salceda R, Riesgo-Escovar JR. Álvarez-Rendón JP, et al. Biomed Res Int. 2018 Apr 24;2018:1417528. doi: 10.1155/2018/1417528. eCollection 2018. Biomed Res Int. 2018. PMID: 29854726 Free PMC article. Review. - Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage.
Kapuria S, Karpac J, Biteau B, Hwangbo D, Jasper H. Kapuria S, et al. PLoS Genet. 2012;8(11):e1003045. doi: 10.1371/journal.pgen.1003045. Epub 2012 Nov 8. PLoS Genet. 2012. PMID: 23144631 Free PMC article. - Identification of gene expression changes associated with the initiation of diapause in the brain of the cotton bollworm, Helicoverpa armigera.
Bao B, Xu WH. Bao B, et al. BMC Genomics. 2011 May 11;12:224. doi: 10.1186/1471-2164-12-224. BMC Genomics. 2011. PMID: 21569297 Free PMC article. - Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony.
Foster KG, Fingar DC. Foster KG, et al. J Biol Chem. 2010 May 7;285(19):14071-7. doi: 10.1074/jbc.R109.094003. Epub 2010 Mar 15. J Biol Chem. 2010. PMID: 20231296 Free PMC article. Review.
References
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. - PubMed
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. - PubMed
- Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25:6384–6391. - PubMed
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous