Regulation of apoptosis by the unfolded protein response - PubMed (original) (raw)
Regulation of apoptosis by the unfolded protein response
Andrew Fribley et al. Methods Mol Biol. 2009.
Abstract
In eukaryotic cells, the endoplasmic reticulum (ER) serves many specialized functions including bio-synthesis and assembly of membrane and secretory proteins, calcium storage and production of lipids and sterols. As a plant for protein folding and posttranslational modification, the ER provides stringent quality control systems to ensure that only correctly folded proteins exit the ER and unfolded or misfolded proteins are retained and ultimately degraded. Biochemical, physiological, and pathological stimuli that interfere with ER function can disrupt ER homeostasis, impose stress to the ER, and subsequently cause accumulation of unfolded or misfolded proteins in the ER lumen. To deal with accumulation of unfolded or misfolded proteins, the cell has evolved highly specific signaling pathways collectively called the "unfolded protein response" (UPR) to restore normal ER functions. However, if the overload of unfolded or misfolded proteins in the ER is not resolved, the prolonged UPR will induce ER stress-associated programmed cell death, apoptosis, to protect the organism by removing the stressed cells. In this chapter, we summarize our current understanding of UPR-induced apoptosis and various methods to detect ER stress and apoptosis in mammalian cells.
Figures
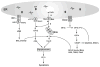
Fig. 1
The apoptotic pathways regulated by the UPR. In response to ER stress, PERK, and IRE1α are activated through their homodimerization and autophosphorylation. Activated PERK phosphorylates translation initiation factor eIF2α, which can selectively induce expression of proapoptotic transcription factors ATF4 and CHOP. CHOP induces expression of numerous proapoptotic factors including DR5, TRB3, CAVI, and BCL2 family proteins. CHOP can also induce expression of GADD34 and ERO1α, leading to apoptosis by increasing protein synthesis and oxidation in the ER of stressed cells. On activation of the UPR, IRE1α serves as a scaffold protein to form a complex with TRAF2 and ASK1, which subsequently activates the JNK-mediated apoptotic pathway. Furthermore, ER stress can induce BAX and BAK localization and oligomerization at the ER, which promotes calcium release from the ER to the cytosol. Increased cytosolic calcium concentration stimulates calcium uptake into the mitochondrial matrix, which can result in depolarization of the inner membrane and transition of the outer membrane permeability pore. This causes cytochrome c release and Apaf-1-dependent activation of the apoptosome leading to apoptosis. In addition, ER stress may also promote dissociation of TRAF2 from ER membrane-resident procaspase-12, allowing caspase-12 activation to mediate apoptosis. Mito Mitochondria, Cyt-c Cytochrome c, pCP12 Procaspase-12, CP12 Caspase-12, CP9 Caspase-9, CP-3 Caspase-3.
Similar articles
- Endoplasmic reticulum stress in liver disease.
Malhi H, Kaufman RJ. Malhi H, et al. J Hepatol. 2011 Apr;54(4):795-809. doi: 10.1016/j.jhep.2010.11.005. Epub 2010 Nov 13. J Hepatol. 2011. PMID: 21145844 Free PMC article. Review. - Enterovirus 3A protein disrupts endoplasmic reticulum homeostasis through interaction with GBF1.
Hirano J, Hayashi T, Kitamura K, Nishimura Y, Shimizu H, Okamoto T, Okada K, Uemura K, Yeh MT, Ono C, Taguwa S, Muramatsu M, Matsuura Y. Hirano J, et al. J Virol. 2024 Jul 23;98(7):e0081324. doi: 10.1128/jvi.00813-24. Epub 2024 Jun 21. J Virol. 2024. PMID: 38904364 Free PMC article. - A review of the mammalian unfolded protein response.
Chakrabarti A, Chen AW, Varner JD. Chakrabarti A, et al. Biotechnol Bioeng. 2011 Dec;108(12):2777-93. doi: 10.1002/bit.23282. Epub 2011 Aug 9. Biotechnol Bioeng. 2011. PMID: 21809331 Free PMC article. Review. - Endoplasmic reticulum stress induced autophagy in cancer and its potential interactions with apoptosis and ferroptosis.
Liao H, Liu S, Ma Q, Huang H, Goel A, Torabian P, Mohan CD, Duan C. Liao H, et al. Biochim Biophys Acta Mol Cell Res. 2025 Jan;1872(1):119869. doi: 10.1016/j.bbamcr.2024.119869. Epub 2024 Oct 26. Biochim Biophys Acta Mol Cell Res. 2025. PMID: 39490702 Review. - Arabidopsis Bax inhibitor-1: A rheostat for ER stress-induced programmed cell death.
Watanabe N, Lam E. Watanabe N, et al. Plant Signal Behav. 2008 Aug;3(8):564-6. doi: 10.4161/psb.3.8.5709. Plant Signal Behav. 2008. PMID: 19704470 Free PMC article.
Cited by
- The ClpP activator ONC-212 (TR-31) inhibits BCL2 and B-cell receptor signaling in CLL.
Fatima N, Shen Y, Crassini K, Iwanowicz EJ, Lang H, Karanewsky DS, Christopherson RI, Mulligan SP, Best OG. Fatima N, et al. EJHaem. 2021 Jan 14;2(1):81-93. doi: 10.1002/jha2.160. eCollection 2021 Feb. EJHaem. 2021. PMID: 35846080 Free PMC article. - Esophageal cancer stem cells reduce hypoxia-induced apoptosis by inhibiting the GRP78-perk-eIF2α-ATF4-CHOP pathway in vitro.
Lin R, Ma M, Han B, Zheng Y, Wang Y, Zhou Y. Lin R, et al. J Gastrointest Oncol. 2023 Aug 31;14(4):1669-1693. doi: 10.21037/jgo-23-462. Epub 2023 Aug 9. J Gastrointest Oncol. 2023. PMID: 37720449 Free PMC article. - Calcium Contributes to the Cytotoxic Interaction Between Diclofenac and Cytokines.
Maiuri AR, Breier AB, Turkus JD, Ganey PE, Roth RA. Maiuri AR, et al. Toxicol Sci. 2016 Feb;149(2):372-84. doi: 10.1093/toxsci/kfv249. Epub 2015 Nov 24. Toxicol Sci. 2016. PMID: 26609140 Free PMC article. - Expanding the clinical spectrum of PPP3CA variants - alternative isoforms matter.
Castiglioni S, Pezzoli L, Pezzani L, Lettieri A, Di Fede E, Cereda A, Ancona S, Gallina A, Colombo EA, Parodi C, Grazioli P, Taci E, Milani D, Iascone M, Massa V, Gervasini C. Castiglioni S, et al. Orphanet J Rare Dis. 2024 Dec 20;19(1):481. doi: 10.1186/s13023-024-03507-0. Orphanet J Rare Dis. 2024. PMID: 39707491 Free PMC article. - Single-Cell RNA-Seq Analysis Reveals Lung Epithelial Cell Type-Specific Responses to HDM and Regulation by Tet1.
Zhu T, Brown AP, Cai LP, Quon G, Ji H. Zhu T, et al. Genes (Basel). 2022 May 14;13(5):880. doi: 10.3390/genes13050880. Genes (Basel). 2022. PMID: 35627266 Free PMC article.
References
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. - PubMed
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
- Schroder M, Kaufman RJ. The Mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. - PubMed
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL057346/HL/NHLBI NIH HHS/United States
- DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173-11/HL/NHLBI NIH HHS/United States
- HL052173/HL/NHLBI NIH HHS/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173-12/HL/NHLBI NIH HHS/United States
- HL057346/HL/NHLBI NIH HHS/United States
- R01 DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials