Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors - PubMed (original) (raw)
Review
Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors
Deborah A Finn et al. Horm Behav. 2010 Jan.
Abstract
Alcoholism is a complex disorder that represents an important contributor to health problems worldwide and that is difficult to encompass with a single preclinical model. Additionally, alcohol (ethanol) influences the function of many neurotransmitter systems, with the interaction at gamma-aminobutyric acid(A) (GABA(A)) receptors being integral for ethanol's reinforcing and several withdrawal-related effects. Given that some steroid derivatives exert rapid membrane actions as potent positive modulators of GABA(A) receptors and exhibit a similar pharmacological profile to that of ethanol, studies in the laboratory manipulated GABAergic steroid levels and determined the impact on ethanol's rewarding- and withdrawal-related effects. Manipulations focused on the progesterone metabolite allopregnanolone (ALLO), since it is the most potent endogenous GABAergic steroid identified. The underlying hypothesis is that fluctuations in GABAergic steroid levels (and the resultant change in GABAergic inhibitory tone) alter sensitivity to ethanol, leading to changes in the positive motivational or withdrawal-related effects of ethanol. This review describes results that emphasize sex differences in the effects of ALLO and the manipulation of its biosynthesis on alcohol reward-versus withdrawal-related behaviors, with females being less sensitive to the modulatory effects of ALLO on ethanol-drinking behaviors but more sensitive to some steroid manipulations on withdrawal-related behaviors. These findings imply the existence of sex differences in the sensitivity of GABA(A) receptors to GABAergic steroids within circuits relevant to alcohol reward versus withdrawal. Thus, sex differences in the modulation of GABAergic neurosteroids may be an important consideration in understanding and developing therapeutic interventions in alcoholics.
2009 Elsevier Inc. All rights reserved.
Figures
Figure 1. The biosynthesis of select steroids with genomic and non-genomic effects
Depicted is the biosynthetic pathway for ALLO, 5α-THDOC, and androstanediol, which are potent positive modulators of GABAA receptors and are formed from the two step reduction of the parent steroids, progesterone, deoxycorticosterone, and testosterone, respectively. The rate-limiting enzyme in GABAergic steroid biosynthesis is 5a-reductase. Also depicted are the sulfated derivatives of pregnenolone and DHEA, which are negative modulators of GABAA receptors, and corticosterone, which has been shown to have excitatory effects. The broken lines indicate that 17-OH pregnenolone and 17-OH progesterone are omitted from the figure in the formation of DHEA and androstenedione, respectively.
Abbreviations:
DHEA, dehydroepiandrosterone; DHDOC, dihydrodeoxycorticosterone; THDOC, tetrahydrodeoxycorticosterone; DHP, dihydroprogesterone; DHT, dihydrotestosterone; 3α-HSD, 3α-hydroxysteroid dehydrogenase. (Adapted from Finn et al., 2006a)
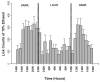
Figure 2. Lickometer assessment of continuous access ethanol drinking patterns
Consumption of 10% v/v ethanol (10E) versus water was examined in male C57BL/6 mice with the use of lickometers, an experimental method that allows for the analysis of self-administration behavior at a fine or microstructure levels by recording individual licks. Based on the hourly distribution, studies examining neurosteroid effects on 10E intake focused on 2 hr limited access sessions, beginning 2-3 hrs after lights out. Values are the mean ± SEM for 24 mice.
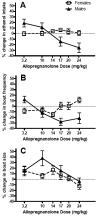
Figure 3. Sex differences in ALLO modulation of (A) ethanol intake, (B) bout frequency, and (C) bout size
All data are presented as % change from baseline values derived from vehicle treatment throughout the dose-response assessment, and are reported as the mean ± SEM of 18 male and 24 female mice. The dashed line depicts the within-subject baseline values for each experimental variable. Graphs depict transformations of previously published data (Ford et al., 2005b, 2008).
Figure 4. Sex differences in FIN modulation of (A) ethanol intake, (B) bout frequency, and (C) bout size
All data are presented as % change from baseline values derived from vehicle treatment prior to acute FIN pretreatment (3-days), and are reported as the mean ± SEM of 16 male and 14 female mice. The solid line depicts within-subject baseline values for each experimental variable. Graphs depict transformations of previously published data (Ford et al., 2005a, 2008).
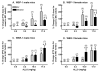
FIGURE 5. Genotype (but no sex) difference in the change in sensitivity to the anticonvulsant effect of ALLO, measured by the percent change in pentylenetetrazol (PTZ) threshold dose for onset to MC twitch, in (A) WSP male, (B) WSP female, (C) WSR male, and (D) WSR female mice
PTZ was administered at 20 min post-injection of ALLO or vehicle. Since ethanol withdrawal significantly decreased the PTZ dose, these data were transformed to % change in PTZ threshold dose (i.e., for each line, sex, treatment and steroid group, the % change was calculated for each animal as the change from the mean for the respective vehicle-injected group – air or ethanol). An increase in PTZ threshold dose following ALLO injection indicated that the dose of ALLO was anticonvulsant. Values represent the mean (± SEM) for the number of animals in parentheses. Data (from replicate-1 animals) were adapted from previously published data (Beckley et al., 2008; Finn et al., 2006b). +P < 0.10, *P < 0.05 vs. respective air-exposed mice #P < 0.05, §P < 0.01 vs. respective vehicle-injected mice
Figure 6. Hypothetical model for interaction of alcohol intoxication- versus alcohol withdrawal-related effects on endogenous ALLO levels and brain excitability
The effect of acute intoxication or alcohol withdrawal on endogenous ALLO levels is depicted by the solid line, while the subsequent change in brain excitability is depicted by the dashed line. In general, the impact of endogenous neurosteroid tone on GABAA receptor-mediated inhibition exhibits an inverse relationship on brain excitability (i.e., ↑ ALLO = ↓ excitation; ↓ ALLO = ↑ excitation). Disparate influences of alcohol intoxication and withdrawal on ALLO levels, in conjunction with differential alterations in GABAA receptor plasticity within discrete brain regions, likely underly sex differences in the effects of GABAergic neurosteroid manipulations on measures of alcohol self-administration and withdrawal.
References
- Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J. Pharmacol. Exp. Ther. 2007;320:427–436. - PubMed
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. - PubMed
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br. J. Psychiatry. 1978;133:1–14. - PubMed
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur. J. Pharmacol. 1999;384:R1–R2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA012439/AA/NIAAA NIH HHS/United States
- AA12439/AA/NIAAA NIH HHS/United States
- P50 AA010760/AA/NIAAA NIH HHS/United States
- F31 AA017019/AA/NIAAA NIH HHS/United States
- F31 MH081560/MH/NIMH NIH HHS/United States
- P60 AA010760/AA/NIAAA NIH HHS/United States
- K01 AA016849/AA/NIAAA NIH HHS/United States
- KO1AA016849/AA/NIAAA NIH HHS/United States
- AA10760/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical